Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes
- PMID: 22586586
- PMCID: PMC3402323
- DOI: 10.2337/db11-1437
Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes
Abstract
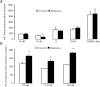
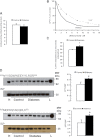
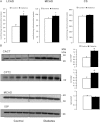
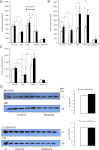
Mitochondrial reactive oxygen species (ROS) cause kidney damage in diabetes. We investigated the source and site of ROS production by kidney cortical tubule mitochondria in streptozotocin-induced type 1 diabetes in rats. In diabetic mitochondria, the increased amounts and activities of selective fatty acid oxidation enzymes is associated with increased oxidative phosphorylation and net ROS production with fatty acid substrates (by 40% and 30%, respectively), whereas pyruvate oxidation is decreased and pyruvate-supported ROS production is unchanged. Oxidation of substrates that donate electrons at specific sites in the electron transport chain (ETC) is unchanged. The increased maximal production of ROS with fatty acid oxidation is not affected by limiting the electron flow from complex I into complex III. The maximal capacity of the ubiquinol oxidation site in complex III in generating ROS does not differ between the control and diabetic mitochondria. In conclusion, the mitochondrial ETC is neither the target nor the site of ROS production in kidney tubule mitochondria in short-term diabetes. Mitochondrial fatty acid oxidation is the source of the increased net ROS production, and the site of electron leakage is located proximal to coenzyme Q at the electron transfer flavoprotein that shuttles electrons from acyl-CoA dehydrogenases to coenzyme Q.
Figures
References
-
- USRDS . USRDS: the United States Renal Data System. Am J Kidney Dis 2003;42(6 Suppl. 5):1–230 - PubMed
-
- Spencer MW, Mühlfeld AS, Segerer S, et al. . Hyperglycemia and hyperlipidemia act synergistically to induce renal disease in LDL receptor-deficient BALB mice. Am J Nephrol 2004;24:20–31 - PubMed
-
- Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498–1509 - PubMed
-
- Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem 2002;277:18919–18927 - PubMed
-
- Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 2006;55:2502–2509 - PubMed

