Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex
- PMID: 22586587
- PMCID: PMC3379648
- DOI: 10.2337/db11-1399
Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex
Abstract
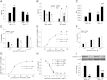
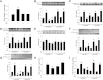
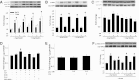
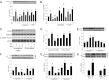
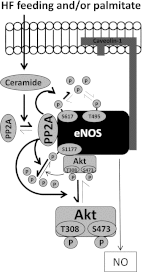
Vascular dysfunction that accompanies obesity and insulin resistance may be mediated by lipid metabolites. We sought to determine if vascular ceramide leads to arterial dysfunction and to elucidate the underlying mechanisms. Pharmacological inhibition of de novo ceramide synthesis, using the Ser palmitoyl transferase inhibitor myriocin, and heterozygous deletion of dihydroceramide desaturase prevented vascular dysfunction and hypertension in mice after high-fat feeding. These findings were recapitulated in isolated arteries in vitro, confirming that ceramide impairs endothelium-dependent vasorelaxation in a tissue-autonomous manner. Studies in endothelial cells reveal that de novo ceramide biosynthesis induced protein phosphatase 2A (PP2A) association directly with the endothelial nitric oxide synthase (eNOS)/Akt/Hsp90 complex that was concurrent with decreased basal and agonist-stimulated eNOS phosphorylation. PP2A attenuates eNOS phosphorylation by preventing phosphorylation of the pool of Akt that colocalizes with eNOS and by dephosphorylating eNOS. Ceramide decreased the association between PP2A and the predominantly cytosolic inhibitor 2 of PP2A. We conclude that ceramide mediates obesity-related vascular dysfunction by a mechanism that involves PP2A-mediated disruption of the eNOS/Akt/Hsp90 signaling complex. These results provide important insight into a pathway that represents a novel target for reversing obesity-related vascular dysfunction.
Figures




References
-
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007 [article online], 2008. Atlanta, GA, U.S. Department of Health and Human Services. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf Accessed August 2011
-
- Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003;108:1527–1532 - PubMed
-
- Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 2005;25:989–994 - PubMed
-
- Edirisinghe I, McCormick Hallam K, Kappagoda CT. Effect of fatty acids on endothelium-dependent relaxation in the rabbit aorta. Clin Sci (Lond) 2006;111:145–151 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

