Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer
- PMID: 22586682
- PMCID: PMC3353724
- DOI: 10.1158/2159-8290.CD-11-0214
Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer
Abstract
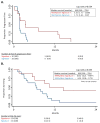
Epigenetic alterations are strongly associated with the development of cancer. We conducted a phase I/II trial of combined epigenetic therapy with azacitidine and entinostat, inhibitors of DNA methylation and histone deacetylation, respectively, in extensively pretreated patients with recurrent metastatic non-small cell lung cancer. This therapy is well tolerated, and objective responses were observed, including a complete response and a partial response in a patient who remains alive and without disease progression approximately 2 years after completing protocol therapy. Median survival in the entire cohort was 6.4 months (95% CI 3.8-9.2), comparing favorably with existing therapeutic options. Demethylation of a set of 4 epigenetically silenced genes known to be associated with lung cancer was detectable in serial blood samples in these patients and was associated with improved progression-free (P = 0.034) and overall survival (P = 0.035). Four of 19 patients had major objective responses to subsequent anticancer therapies given immediately after epigenetic therapy.
Significance: This study demonstrates that combined epigenetic therapy with low-dose azacitidine and entinostat results in objective, durable responses in patients with solid tumors and defines a blood-based biomarker that correlates with clinical benefit.
Conflict of interest statement
Potential conflicts of interest: RAJ and CMR have consulted for Syndax. JGH has consulted for MDx Health, JGH and MVB have research support from MDxHealth, and JGH, SBB, and MVB hold a patent licensed to MDx Health. These arrangements are managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures



Comment in
-
A combined epigenetic therapy equals the efficacy of conventional chemotherapy in refractory advanced non-small cell lung cancer.Cancer Discov. 2011 Dec;1(7):557-9. doi: 10.1158/2159-8290.CD-11-0271. Epub 2011 Nov 9. Cancer Discov. 2011. PMID: 22586680
References
-
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. - PubMed
-
- Glover AB, Leyland-Jones BR, Chun HG, Davies B, Hoth DF. Azacitidine: 10 years later. Cancer Treat Rep. 1987;71:737–46. - PubMed
-
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

