Deconstructing pancreas developmental biology
- PMID: 22587935
- PMCID: PMC3367550
- DOI: 10.1101/cshperspect.a012401
Deconstructing pancreas developmental biology
Abstract
The relentless nature and increasing prevalence of human pancreatic diseases, in particular, diabetes mellitus and adenocarcinoma, has motivated further understanding of pancreas organogenesis. The pancreas is a multifunctional organ whose epithelial cells govern a diversity of physiologically vital endocrine and exocrine functions. The mechanisms governing the birth, differentiation, morphogenesis, growth, maturation, and maintenance of the endocrine and exocrine components in the pancreas have been discovered recently with increasing tempo. This includes recent studies unveiling mechanisms permitting unexpected flexibility in the developmental potential of immature and mature pancreatic cell subsets, including the ability to interconvert fates. In this article, we describe how classical cell biology, genetic analysis, lineage tracing, and embryological investigations are being complemented by powerful modern methods including epigenetic analysis, time-lapse imaging, and flow cytometry-based cell purification to dissect fundamental processes of pancreas development.
Figures
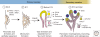

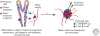

References
-
- Ahlgren U, Jonsson J, Edlund H. 1996. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF/PDX1-deficient mice. Development 122: 1409–1416 - PubMed
-
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H 1999. Notch signalling controls pancreatic cell differentiation. Nature 400: 877–881 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
