Mycobacterium tuberculosis and Clostridium difficille interactomes: demonstration of rapid development of computational system for bacterial interactome prediction
- PMID: 22587966
- PMCID: PMC3353838
- DOI: 10.1186/2042-5783-2-4
Mycobacterium tuberculosis and Clostridium difficille interactomes: demonstration of rapid development of computational system for bacterial interactome prediction
Abstract
Background: Protein-protein interaction (PPI) networks (interactomes) of most organisms, except for some model organisms, are largely unknown. Experimental methods including high-throughput techniques are highly resource intensive. Therefore, computational discovery of PPIs can accelerate biological discovery by presenting "most-promising" pairs of proteins that are likely to interact. For many bacteria, genome sequence, and thereby genomic context of proteomes, is readily available; additionally, for some of these proteomes, localization and functional annotations are also available, but interactomes are not available. We present here a method for rapid development of computational system to predict interactome of bacterial proteomes. While other studies have presented methods to transfer interologs across species, here, we propose transfer of computational models to benefit from cross-species annotations, thereby predicting many more novel interactions even in the absence of interologs. Mycobacterium tuberculosis (Mtb) and Clostridium difficile (CD) have been used to demonstrate the work.
Results: We developed a random forest classifier over features derived from Gene Ontology annotations and genetic context scores provided by STRING database for predicting Mtb and CD interactions independently. The Mtb classifier gave a precision of 94% and a recall of 23% on a held out test set. The Mtb model was then run on all the 8 million protein pairs of the Mtb proteome, resulting in 708 new interactions (at 94% expected precision) or 1,595 new interactions at 80% expected precision. The CD classifier gave a precision of 90% and a recall of 16% on a held out test set. The CD model was run on all the 8 million protein pairs of the CD proteome, resulting in 143 new interactions (at 90% expected precision) or 580 new interactions (at 80% expected precision). We also compared the overlap of predictions of our method with STRING database interactions for CD and Mtb and also with interactions identified recently by a bacterial 2-hybrid system for Mtb. To demonstrate the utility of transfer of computational models, we made use of the developed Mtb model and used it to predict CD protein-pairs. The cross species model thus developed yielded a precision of 88% at a recall of 8%. To demonstrate transfer of features from other organisms in the absence of feature-based and interaction-based information, we transferred missing feature values from Mtb orthologs into the CD data. In transferring this data from orthologs (not interologs), we showed that a large number of interactions can be predicted.
Conclusions: Rapid discovery of (partial) bacterial interactome can be made by using existing set of GO and STRING features associated with the organisms. We can make use of cross-species interactome development, when there are not even sufficient known interactions to develop a computational prediction system. Computational model of well-studied organism(s) can be employed to make the initial interactome prediction for the target organism. We have also demonstrated successfully, that annotations can be transferred from orthologs in well-studied organisms enabling accurate predictions for organisms with no annotations. These approaches can serve as building blocks to address the challenges associated with feature coverage, missing interactions towards rapid interactome discovery for bacterial organisms.
Availability: The predictions for all Mtb and CD proteins are made available at: http://severus.dbmi.pitt.edu/TB and http://severus.dbmi.pitt.edu/CD respectively for browsing as well as for download.
Figures

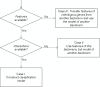


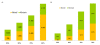
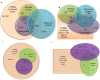


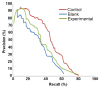
Similar articles
-
Computational Network Inference for Bacterial Interactomics.mSystems. 2022 Apr 26;7(2):e0145621. doi: 10.1128/msystems.01456-21. Epub 2022 Mar 30. mSystems. 2022. PMID: 35353009 Free PMC article. Review.
-
Comparative analysis and assessment of M. tuberculosis H37Rv protein-protein interaction datasets.BMC Genomics. 2011 Nov 30;12 Suppl 3(Suppl 3):S20. doi: 10.1186/1471-2164-12-S3-S20. Epub 2011 Nov 30. BMC Genomics. 2011. PMID: 22369691 Free PMC article.
-
Bacterial protein meta-interactomes predict cross-species interactions and protein function.BMC Bioinformatics. 2017 Mar 16;18(1):171. doi: 10.1186/s12859-017-1585-0. BMC Bioinformatics. 2017. PMID: 28298180 Free PMC article.
-
Complementing the Eukaryotic Protein Interactome.PLoS One. 2013 Jun 18;8(6):e66635. doi: 10.1371/journal.pone.0066635. Print 2013. PLoS One. 2013. PMID: 23825550 Free PMC article.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
Cited by
-
Research prioritization through prediction of future impact on biomedical science: a position paper on inference-analytics.Gigascience. 2013 Aug 30;2(1):11. doi: 10.1186/2047-217X-2-11. Gigascience. 2013. PMID: 24001106 Free PMC article.
-
Computational Network Inference for Bacterial Interactomics.mSystems. 2022 Apr 26;7(2):e0145621. doi: 10.1128/msystems.01456-21. Epub 2022 Mar 30. mSystems. 2022. PMID: 35353009 Free PMC article. Review.
-
Identifying Protein Complexes With Clear Module Structure Using Pairwise Constraints in Protein Interaction Networks.Front Genet. 2021 Aug 27;12:664786. doi: 10.3389/fgene.2021.664786. eCollection 2021. Front Genet. 2021. PMID: 34512712 Free PMC article.
-
Comparative genomics of 274 Vibrio cholerae genomes reveals mobile functions structuring three niche dimensions.BMC Genomics. 2014 Aug 5;15(1):654. doi: 10.1186/1471-2164-15-654. BMC Genomics. 2014. PMID: 25096633 Free PMC article.
-
Transferring knowledge of bacterial protein interaction networks to predict pathogen targeted human genes and immune signaling pathways: a case study on M. tuberculosis.BMC Genomics. 2018 Jun 28;19(1):505. doi: 10.1186/s12864-018-4873-9. BMC Genomics. 2018. PMID: 29954330 Free PMC article.
References
-
- Organisation WH. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. 2010.
Grants and funding
LinkOut - more resources
Full Text Sources
