Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films
- PMID: 22588074
- PMCID: PMC3396844
- DOI: 10.1016/j.actbio.2012.05.006
Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films
Abstract
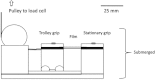
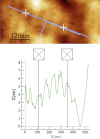
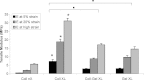
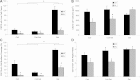
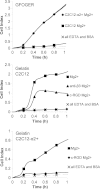
This study focuses on determining the effect of varying the composition and crosslinking of collagen-based films on their physical properties and interaction with myoblasts. Films composed of collagen or gelatin and crosslinked with a carbodiimide were assessed for their surface roughness and stiffness. These samples are significant because they allow variation of physical properties as well as offering different recognition motifs for cell binding. Cell reactivity was determined by the ability of myoblastic C2C12 and C2C12-α2+ cell lines (with different integrin expression) to adhere to and spread on the films. Significantly, crosslinking reduced the cell reactivity of all films, irrespective of their initial composition, stiffness or roughness. Crosslinking resulted in a dramatic increase in the stiffness of the collagen film and also tended to reduce the roughness of the films (R(q) = 0.417 ± 0.035 μm, E = 31 ± 4.4 MPa). Gelatin films were generally smoother and more compliant than comparable collagen films (R(q) = 7.9 ± 1.5 nm, E = 15 ± 3.1 MPa). The adhesion of α2-positive cells was enhanced relative to the parental C2C12 cells on collagen compared with gelatin films. These results indicate that the detrimental effect of crosslinking on cell response may be due to the altered physical properties of the films as well as a reduction in the number of available cell binding sites. Hence, although crosslinking can be used to enhance the mechanical stiffness and reduce the roughness of films, it reduces their capacity to support cell activity and could potentially limit the effectiveness of the collagen-based films and scaffolds.
Copyright © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures







References
-
- Chen Q.-Z., Ali N.N., Lyon A.R., Boccaccini A.R. Biomaterials in cardiac tissue engineering: ten years of research survey. Mater Sci Eng. 2008;59:1–37.
-
- Eschenhagen T., Fink C., Remmers U., Scholz H., Wattchow J., Weil J. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB. 1997;11:683–694. - PubMed
-
- Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1–22. - PubMed
-
- Xiang Z., Liao R., Kelly M.S., Spector M. Collagen–GAG scaffolds grafted onto myocardial infarcts in a rat model: a delivery vehicle for mesenchymal stem cells. Tissue Eng. 2006;12:2467–2478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

