A pooled exploratory analysis of the effect of tumor size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer
- PMID: 22588152
- PMCID: PMC3638870
- DOI: 10.1097/JTO.0b013e31824fe9e6
A pooled exploratory analysis of the effect of tumor size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer
Abstract
Introduction: The staging of node-negative non-small-cell lung cancer is modified in the 7th edition TNM classification. Here, we pool data from the National Cancer Institute of Canada Clinical Trials Group JBR.10 trial and the Cancer and Leukemia Group B-9633 trial to explore the prognostic and predictive effects of the new T-size descriptors and KRAS mutation status.
Methods: Node-negative patients were reclassified as T2a (>3-≤5 cm), T2b (>5-≤7 cm), T3 (>7 cm) or T ≤ 3 cm (≤3 cm, but other T2 characteristics).
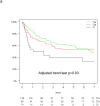
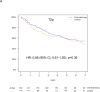
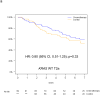
Results: Of 538 eligible patients, 288 (53.5%) were T2a, 111 (21%) T2b, 62 (11.5%) T3, whereas 77 (14%) T≤3 cm were excluded to avoid confounding. KRAS mutations were detected in 104 of 390 patients (27%). T-size was prognostic for disease-free survival (p = 0.03), but borderline for overall survival (OS; p = 0.10), on multivariable analysis. Significant interaction between the prognostic value of KRAS and tumor size was observed for OS (p = 0.01), but not disease-free survival (p = 0.10). There was a nonsignificant trend (p = 0.24) for increased chemotherapy effect on OS with advancing T-size (hazard ratio [HR] T2a 0.90, [0.63-1.30]; T2b 0.69, [0.38-1.24]; and T3 0.57, [0.28-1.17]). The HR for chemotherapy effect on OS in T2a patients with KRAS wild-type tumors was 0.81 (p = 0.36), whereas a trend for detrimental effect was observed in those with mutant tumors (HR 2.11; p = 0.09; interaction p = 0.05). Similar trends were observed in T2b to T3 patients with wild-type (HR 0.86; p = 0.62), and KRAS mutant tumors (HR 1.16; p = 0.74; interaction p = 0.58).
Conclusion: Chemotherapy effect seems to increase with tumor size. However, this small study could not identify subgroups of patients who did or did not derive significant benefit from adjuvant chemotherapy based on T-size or KRAS status.
Figures











References
-
- The International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. - PubMed
-
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. - PubMed
-
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. - PubMed
-
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

