Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(-/-) mice
- PMID: 22588244
- PMCID: PMC3664520
- DOI: 10.1007/s10620-012-2217-1
Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(-/-) mice
Abstract
Background: Soluble epoxide hydrolase (sEH) metabolizes anti-inflammatory epoxyeicosatrienoic acids (EETs) into their much less active dihydroxy derivatives dihydroxyeicosatrienoic acids. Thus, targeting sEH would be important for inflammation.
Aims: To determine whether knockout or inhibition of sEH would attenuate the development of inflammatory bowel disease (IBD) in a mouse model of IBD in IL-10(-/-) mice.
Methods: Either the small molecule sEH inhibitor trans/-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB) or sEH knockout mice were used in combination with IL-10(-/-) mice. t-AUCB was administered to mice in drinking fluid. Extensive histopathologic, immunochemical, and biochemical analyses were performed to evaluate effect of sEH inhibition or deficiency on chronic active inflammation and related mechanism in the bowel.
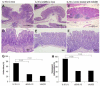
Results: Compared to IL-10 (-/-) mice, sEH inhibition or sEH deficiency in IL-10(-/-) mice resulted in significantly lower incidence of active ulcer formation and transmural inflammation, along with a significant decrease in myeloperoxidase-labeled neutrophil infiltration in the inflamed bowel. The levels of IFN-γ, TNF-α, and MCP-1, as well VCAM-1 and NF-kB/IKK-α signals were significantly decreased as compared to control animals. Moreover, an eicosanoid profile analysis revealed a significant increase in the ratio of EETs/DHET and EpOME/DiOME, and a slightly down-regulation of inflammatory mediators LTB(4) and 5-HETE.
Conclusion: These results indicate that sEH gene deficiency or inhibition reduces inflammatory activities in the IL-10 (-/-) mouse model of IBD, and that sEH inhibitor could be a highly potential in the treatment of IBD.
Figures




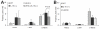
References
-
- Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. - PubMed
-
- Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol. 2007;293(1):F342–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

