Educational paper: do we need neonatal clinical pharmacologists?
- PMID: 22588521
- PMCID: PMC4709249
- DOI: 10.1007/s00431-012-1734-4
Educational paper: do we need neonatal clinical pharmacologists?
Abstract
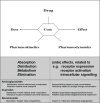
Effective and safe drug administration in young infants should be based on integrated knowledge concerning the evolving physiological characteristics of the infant who will receive the drug and the pharmacokinetic and pharmacodynamic characteristics of a given drug. Consequently, clinical pharmacology in neonates is as dynamic and diverse as the neonates we are entitled to take care of. Even more than median estimates, covariates of variability within the population are of clinical relevance. We aim to illustrate the complexity and the need for neonatal clinical pharmacology based on the gap between current and likely best clinical practice for two commonly administered compounds (aminoglycosides for infection and ibuprofen for patent ductus arteriosus) and one new compound (bevacizumab, to treat threshold retinopathy of prematurity). Progression has been made to render pharmacokinetic studies child size, e.g., low volume samples, optimal study design, and population pharmacokinetics. Challenges to further improve clinical pharmacology in neonates include, when appropriate, the validation of off-patent drug dosing regimens and of infant-tailored formulations. Knowledge integration, i.e., the use of available data to improve current drug use and to predict pharmacokinetics/pharmacodynamics for similar compounds is needed. Development of clinical research networks is helpful to achieve these goals.
Figures
References
-
- Allegaert K, Verbesselt R, Naulaers G, van den Anker JN, Rayyan M, Debeer A, de Hoon J. Developmental pharmacology: neonates are not just small adults…. Acta Clin Belg. 2008;63:16–24. - PubMed
-
- Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–829. - PubMed
-
- Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: general principles. Eur J Pediatr. 2006;165:741–746. - PubMed
-
- Ben-Ari Y. Blocking seizures with the diuretic bumetanide: promises and pitfalls. Epilepsia. 2012;53:394–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous