Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair
- PMID: 22589372
- PMCID: PMC3406600
- DOI: 10.1161/CIRCRESAHA.112.270462
Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair
Abstract
Rationale: Although bone marrow endothelial progenitor cell (EPC)-based therapies improve the symptoms in patients with ischemic heart disease, their limited plasticity and decreased function in patients with existing heart disease limit the full benefit of EPC therapy for cardiac regenerative medicine.
Objective: We hypothesized that reprogramming mouse or human EPCs, or both, using small molecules targeting key epigenetic repressive marks would lead to a global increase in active gene transcription, induce their cardiomyogenic potential, and enhance their inherent angiogenic potential.
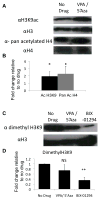
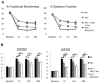
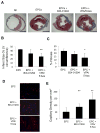
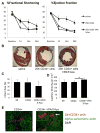
Method and results: Mouse Lin-Sca1(+)CD31(+) EPCs and human CD34(+) cells were treated with inhibitors of DNA methyltransferases (5-Azacytidine), histone deacetylases (valproic acid), and G9a histone dimethyltransferase. A 48-hour treatment led to global increase in active transcriptome, including the reactivation of pluripotency-associated and cardiomyocyte-specific mRNA expression, whereas endothelial cell-specific genes were significantly upregulated. When cultured under appropriate differentiation conditions, reprogrammed EPCs showed efficient differentiation into cardiomyocytes. Treatment with epigenetic-modifying agents show marked increase in histone acetylation on cardiomyocyte and pluripotent cell-specific gene promoters. Intramyocardial transplantation of reprogrammed mouse and human EPCs in an acute myocardial infarction mouse model showed significant improvement in ventricular functions, which was histologically supported by their de novo cardiomyocyte differentiation and increased capillary density and reduced fibrosis. Importantly, cell transplantation was safe and did not form teratomas.
Conclusions: Taken together, our results suggest that epigenetically reprogrammed EPCs display a safe, more plastic phenotype and improve postinfarct cardiac repair by both neocardiomyogenesis and neovascularization.
Figures



Comment in
-
Created equal? The many facets of cell reprogramming.Circ Res. 2012 Jul 6;111(2):152-5. doi: 10.1161/CIRCRESAHA.112.272526. Circ Res. 2012. Retraction in: Circ Res. 2019 Feb 15;124(4):e27. doi: 10.1161/RES.0000000000000251. PMID: 22773422 Free PMC article. Retracted. No abstract available.
References
-
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (topcare-ami): Mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. - PubMed
-
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part ii: Cell-based therapies. Circulation. 2004;109:2692–2697. - PubMed
-
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part i: Angiogenic cytokines. Circulation. 2004;109:2487–2491. - PubMed
-
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the repair-ami trial. Eur Heart J. 2006;27:2775–2783. - PubMed
-
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

