ER stress is involved in T17M rhodopsin-induced retinal degeneration
- PMID: 22589437
- PMCID: PMC3390184
- DOI: 10.1167/iovs.11-9235
ER stress is involved in T17M rhodopsin-induced retinal degeneration
Abstract
Purpose: The human rhodopsin (Rho) mutation T17M leads to autosomal dominant retinitis pigmentosa (adRP). The goal of our study was to elucidate the role of endoplasmic reticulum (ER) stress in retinal degeneration in hT17M Rho mice and identify potential candidates for adRP gene therapy.
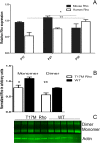
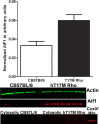
Methods: We used transgenic mice expressing the ER stress-activated indicator (ERAI) and hT17M Rho to evaluate the activation of ER stress responses. Quantitative reverse transcription PCR (qRT-PCR) was used to analyze changes in the expression of 30 unfolded protein response (UPR)-associated genes at P12, 15, 18, 21, and 25. The cytosolic fraction of hT17M Rho retinal cells was used to measure the release of cytochrome C and apoptotic inducing factor-1 (AIF1) by Western blotting. Optical coherence tomography (OCT) analysis was performed for 1-month-old hT17M Rho mice.
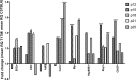
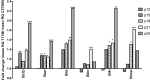
Results: hT17M Rho was localized in the outer nuclear layer (ONL) of T17M(+/-)ERAI(+/-) photoreceptors as well as C57BL/6 retinas injected with AAV-hT17M Rho-GFP. In P15 hT17M Rho retinas, we observed an up-regulation of UPR genes (Atf4, Eif2α, Xbp1, Bip, Canx, and Hsp90), autophagy genes and proapoptotic Bcl2 genes. OCT, and the downregulation of Nrl and Crx gene expression confirmed that cell death occurs in 55% of photoreceptors via the up-regulation of caspase-3 and caspase-12, and the release of AIF1 from the mitochondria.
Conclusions: The ER stress response is involved in retinal degeneration in hT17M Rho mice. The final demise of photoreceptors occurs via apoptosis involving ER stress-associated and mitochondria-induced caspase activation. We identified Atg5, Atg7, Bax, Bid, Bik, and Noxa as potential therapeutic targets for adRP treatment.
Conflict of interest statement
Disclosure:
Figures



References
-
- Dryja TP, McGee TL, Hahn LB, et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990;323:1302–1307 - PubMed
-
- Krebs MP, Holden DC, Joshi P, Clark CL, III, Lee AH, Kaushal S. Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J Mol Biol. 2010;395:1063–1078 - PubMed
-
- Kaushal S, Khorana HG. Structure and function in rhodopsin. 7. Point mutations associated with autosomal dominant retinitis pigmentosa. Biochemistry. 1994;33:6121–6128 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

