Molecular determinants of Gem protein inhibition of P/Q-type Ca2+ channels
- PMID: 22589533
- PMCID: PMC3391154
- DOI: 10.1074/jbc.M111.291872
Molecular determinants of Gem protein inhibition of P/Q-type Ca2+ channels
Abstract
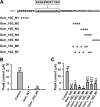
The RGK family of monomeric GTP-binding proteins potently inhibits high voltage-activated Ca(2+) channels. The molecular mechanisms of this inhibition are largely unclear. In Xenopus oocytes, Gem suppresses the activity of P/Q-type Ca(2+) channels on the plasma membrane. This is presumed to occur through direct interactions of one or more Gem inhibitory sites and the pore-forming Ca(v)2.1 subunit in a manner dependent on the Ca(2+) channel subunit β (Ca(v)β). In this study we investigated the molecular determinants in Gem that are critical for this inhibition. Like other RGK proteins, Gem contains a conserved Ras-like core and extended N and C termini. A 12-amino acid fragment in the C terminus was found to be crucial for and sufficient to produce Ca(v)β-dependent inhibition, suggesting that this region forms an inhibitory site. A three-amino acid motif in the core was also found to be critical, possibly forming another inhibitory site. Mutating either site individually did not hamper Gem inhibition, but mutating both sites together completely abolished Gem inhibition without affecting Gem protein expression level or disrupting Gem interaction with Ca(v)2.1 or Ca(v)β. Mutating Gem residues that are crucial for interactions with previously demonstrated RGK modulators such as calmodulin, 14-3-3, and phosphatidylinositol lipids did not significantly affect Gem inhibition. These results suggest that Gem contains two candidate inhibitory sites, each capable of producing full inhibition of P/Q-type Ca(2+) channels.
Figures




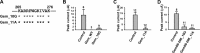




References
-
- Felix R. (2005) Molecular regulation of voltage-gated Ca2+ channels. J. Recept. Signal Transduct. Res. 25, 57–71 - PubMed
-
- Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 - PubMed
-
- Kelly K. (2005) The RGK family. A regulatory tail of small GTP-binding proteins. Trends Cell Biol. 15, 640–643 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

