Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein
- PMID: 22589539
- PMCID: PMC3391160
- DOI: 10.1074/jbc.M112.345918
Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein
Abstract
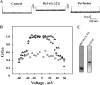
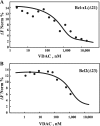
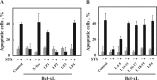
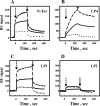
The mitochondrial protein, the voltage-dependent anion channel (VDAC), is implicated in the control of apoptosis, including via its interaction with the pro- and antiapoptotic proteins. We previously demonstrated the direct interaction of Bcl2 with VDAC, leading to reduced channel conductance. VDAC1-based peptides interacted with Bcl2 to prevent its antiapoptotic activity. Here, using a variety of approaches, we show the interaction of the antiapoptotic protein, Bcl-xL, with VDAC1 and reveal that this interaction mediates Bcl-xL protection against apoptosis. C-terminally truncated Bcl-xL(Δ21) interacts with purified VDAC1, as revealed by microscale thermophoresis and as reflected in the reduced channel conductivity of bilayer-reconstituted VDAC1. Overexpression of Bcl-xL prevented staurosporine-induced apoptosis in cells expressing native VDAC1 but not certain VDAC1 mutants. Having identified mutations in VDAC1 that interfere with the Bcl-xL interaction, certain peptides representing VDAC1 sequences, including the N-terminal domain, were designed and generated as recombinant and synthetic peptides. The VDAC1 N-terminal region and two internal sequences were found to bind specifically, and in a concentration- and time-dependent manner, to immobilized Bcl-xL(Δ21), as revealed by surface plasmon resonance. Moreover, expression of the recombinant peptides in cells overexpressing Bcl-xL prevented protection offered by the protein against staurosporine-induced apoptosis. These results point to Bcl-xL acting as antiapoptotic protein, promoting tumor cell survival via binding to VDAC1. These findings suggest that interfering with Bcl-xL binding to the mitochondria by VDAC1-based peptides may serve to induce apoptosis in cancer cells and to potentiate the efficacy of conventional chemotherapeutic agents.
Figures

References
-
- Cory S., Adams J. M. (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2, 647–656 - PubMed
-
- Reed J. C. (2002) Apoptosis-based therapies. Nat. Rev. Drug Discov. 1, 111–121 - PubMed
-
- Youle R. J., Strasser A. (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 - PubMed
-
- Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S. A., Letai A. (2006) Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

