Solid-state NMR reveals a close structural relationship between amyloid-β protofibrils and oligomers
- PMID: 22589542
- PMCID: PMC3391088
- DOI: 10.1074/jbc.M112.367474
Solid-state NMR reveals a close structural relationship between amyloid-β protofibrils and oligomers
Abstract
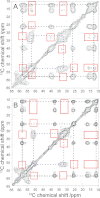
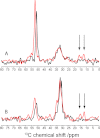
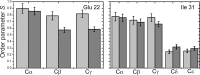
We have studied tertiary contacts in protofibrils and mature fibrils of amyloid-β (Aβ) peptides using solid-state NMR spectroscopy. Although intraresidue contacts between Glu-22 and Ile-31 were found in Aβ protofibrils, these contacts were completely absent in mature Aβ fibrils. This is consistent with the current models of mature Aβ fibrils. As these intramolecular contacts have also been reported in Aβ oligomers, our measurements suggest that Aβ protofibrils are structurally more closely related to oligomers than to mature fibrils. This suggests that some structural alterations have to take place on the pathway from Aβ oligomers/protofibrils to mature fibrils, in agreement with a model that suggests a conversion of intramolecular hydrogen-bonded structures of Aβ oligomers to the intermolecular stabilized mature fibrils (Hoyer, W., Grönwall, C., Jonsson, A., Ståhl, S., and Härd, T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5099-5104).
Figures

References
-
- Finder V. H., Glockshuber R. (2007) Amyloid-βaggregation. Neurodegener. Dis. 4, 13–27 - PubMed
-
- Morgado I., Fändrich M. (2011) Assembly of Alzheimer Aβ peptide into nanostructured amyloid fibrils. Curr. Opin. Colloid Interface Sci. 16, 508–514
-
- Goldsbury C. S., Wirtz S., Müller S. A., Sunderji S., Wicki P., Aebi U., Frey P. (2000) Studies on the in vitro assembly of Aβ(1–40): implications for the search for Aβ fibril formation inhibitors. J. Struct. Biol. 130, 217–231 - PubMed
-
- Harper J. D., Wong S. S., Lieber C. M., Lansbury P. T. (1997) Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 4, 119–125 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

