The NDR/LATS kinase Cbk1 controls the activity of the transcriptional regulator Bcr1 during biofilm formation in Candida albicans
- PMID: 22589718
- PMCID: PMC3349750
- DOI: 10.1371/journal.ppat.1002683
The NDR/LATS kinase Cbk1 controls the activity of the transcriptional regulator Bcr1 during biofilm formation in Candida albicans
Abstract
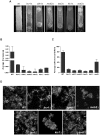
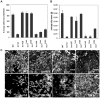
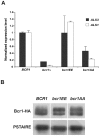
In nature, many microorganisms form specialized complex, multicellular, surface-attached communities called biofilms. These communities play critical roles in microbial pathogenesis. The fungal pathogen Candida albicans is associated with catheter-based infections due to its ability to establish biofilms. The transcription factor Bcr1 is a master regulator of C. albicans biofilm development, although the full extent of its regulation remains unknown. Here, we report that Bcr1 is a phosphoprotein that physically interacts with the NDR kinase Cbk1 and undergoes Cbk1-dependent phosphorylation. Mutating the two putative Cbk1 phosphoacceptor residues in Bcr1 to alanine markedly impaired Bcr1 function during biofilm formation and virulence in a mouse model of disseminated candidiasis. Cells lacking Cbk1, or any of its upstream activators, also had reduced biofilm development. Notably, mutating the two putative Cbk1 phosphoacceptor residues in Bcr1 to glutamate in cbk1Δ cells upregulated the transcription of Bcr1-dependent genes and partially rescued the biofilm defects of a cbk1Δ strain. Therefore, our data uncovered a novel role of the NDR/LATS kinase Cbk1 in the regulation of biofilm development through the control of Bcr1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002;5:608–611. - PubMed
-
- Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. - PubMed
-
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. - PubMed
-
- Verstrepen KJ, Reynolds TB, Fink GR. Origins of variation in the fungal cell surface. Nat Rev Microbiol. 2004;2:533–540. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

