Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy
- PMID: 22589721
- PMCID: PMC3349755
- DOI: 10.1371/journal.ppat.1002689
Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy
Abstract
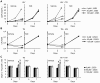
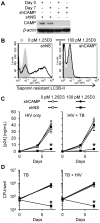
Low vitamin D levels in human immunodeficiency virus type-1 (HIV) infected persons are associated with more rapid disease progression and increased risk for Mycobacterium tuberculosis infection. We have previously shown that 1α,25-dihydroxycholecalciferol (1,25D3), the active form of vitamin D, inhibits HIV replication in human macrophages through the induction of autophagy. In this study, we report that physiological concentrations of 1,25D3 induce the production of the human cathelicidin microbial peptide (CAMP) and autophagic flux in HIV and M. tuberculosis co-infected human macrophages which inhibits mycobacterial growth and the replication of HIV. Using RNA interference for Beclin-1 and the autophagy-related 5 homologue, combined with the chemical inhibitors of autophagic flux, bafilomycin A₁, an inhibitor of autophagosome-lysosome fusion and subsequent acidification, and SID 26681509 an inhibitor of the lysosome hydrolase cathepsin L, we show that the 1,25D3-mediated inhibition of HIV replication and mycobacterial growth during single infection or dual infection is dependent not only upon the induction of autophagy, but also through phagosomal maturation. Moreover, through the use of RNA interference for CAMP, we demonstrate that cathelicidin is essential for the 1,25D3 induced autophagic flux and inhibition of HIV replication and mycobacterial growth. The present findings provide a biological explanation for the benefits and importance of vitamin D sufficiency in HIV and M. tuberculosis-infected persons, and provide new insights into novel approaches to prevent and treat HIV infection and related opportunistic infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- de Noronha AL, Bafica A, Nogueira L, Barral A, Barral-Netto M. Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathol Res Pract. 2008;204:155–161. - PubMed
-
- Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–646. - PubMed
-
- Safi H, Gormus BJ, Didier PJ, Blanchard JL, Lakey DL, et al. Spectrum of manifestations of Mycobacterium tuberculosis infection in primates infected with SIV. AIDS Res Hum Retroviruses. 2003;19:585–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

