Histone h1 depletion impairs embryonic stem cell differentiation
- PMID: 22589736
- PMCID: PMC3349736
- DOI: 10.1371/journal.pgen.1002691
Histone h1 depletion impairs embryonic stem cell differentiation
Abstract
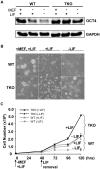
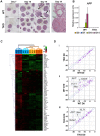
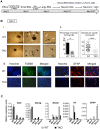
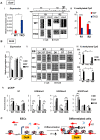
Pluripotent embryonic stem cells (ESCs) are known to possess a relatively open chromatin structure; yet, despite efforts to characterize the chromatin signatures of ESCs, the role of chromatin compaction in stem cell fate and function remains elusive. Linker histone H1 is important for higher-order chromatin folding and is essential for mammalian embryogenesis. To investigate the role of H1 and chromatin compaction in stem cell pluripotency and differentiation, we examine the differentiation of embryonic stem cells that are depleted of multiple H1 subtypes. H1c/H1d/H1e triple null ESCs are more resistant to spontaneous differentiation in adherent monolayer culture upon removal of leukemia inhibitory factor. Similarly, the majority of the triple-H1 null embryoid bodies (EBs) lack morphological structures representing the three germ layers and retain gene expression signatures characteristic of undifferentiated ESCs. Furthermore, upon neural differentiation of EBs, triple-H1 null cell cultures are deficient in neurite outgrowth and lack efficient activation of neural markers. Finally, we discover that triple-H1 null embryos and EBs fail to fully repress the expression of the pluripotency genes in comparison with wild-type controls and that H1 depletion impairs DNA methylation and changes of histone marks at promoter regions necessary for efficiently silencing pluripotency gene Oct4 during stem cell differentiation and embryogenesis. In summary, we demonstrate that H1 plays a critical role in pluripotent stem cell differentiation, and our results suggest that H1 and chromatin compaction may mediate pluripotent stem cell differentiation through epigenetic repression of the pluripotency genes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;28:1079–1088. - PubMed
-
- Ahmed K, Dehghani H, Rugg-Gunn P, Fussner E, Rossant J, et al. Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo. PLoS ONE. 2010;5:e10531. doi: 10.1371/journal.pone.0010531. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

