Triprolidinium dichloranilate-chloranilic acid-methanol-water (2/1/2/2)
- PMID: 22589908
- PMCID: PMC3343999
- DOI: 10.1107/S1600536812010136
Triprolidinium dichloranilate-chloranilic acid-methanol-water (2/1/2/2)
Abstract
In the triprolidinium cation of the title compound {systematic name: 2-[1-(4-methyl-phen-yl)-3-(pyrrolidin-1-ium-1-yl)prop-1-en-1-yl]pyridin-1-ium bis-(2,5-dichloro-4-hy-droxy-3,6-dioxo-cyclo-hexa-1,4-dien-1-olate)-2,5-dichloro-3,6-dihy-droxy-cyclo-hexa-2,5-diene-1,4-dione-methanol-water (2/1/2/2)}, C(19)H(24)N(2) (2+)·2C(6)HCl(2)O(4) (-)·0.5C(6)H(2)Cl(2)O(4)·CH(3)OH·H(2)O, the N atoms on both the pyrrolidine and pyridine groups are protonated. The neutral chloranilic acid mol-ecule is on an inversion symmetry element and its hy-droxy H atoms are disordered over two positions with site-occupancy factors of 0.53 (6) and 0.47 (6). The methanol solvent mol-ecule is disordered over two positions in a 0.836 (4):0.164 (4) ratio. In the crystal, N-H⋯O, O-H⋯O and C-H⋯O inter-actions link the components. The crystal structure also features π-π inter-actions between the benzene rings [centroid-centroid distances = 3.5674 (15), 3.5225 (15) and 3.6347 (15) Å].
Figures
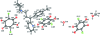

References
-
- Adam, M. S., Parkin, A., Thomas, L. H. & Wilson, C. C. (2010). CrystEngComm, 12, 917–924.
-
- Agilent (2011). CrysAlis PRO and CrysAlis RED Agilent Technologies Ltd, Yarnton, England.
-
- Al-Attas, A. S., Habeeb, M. M. & Al-Raimi, D. S. (2009). J. Mol. Struct. 928, 158–170.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
