1-(Anthracen-1-yl)pyrrolidine-2,5-dione
- PMID: 22590374
- PMCID: PMC3344612
- DOI: 10.1107/S160053681201536X
1-(Anthracen-1-yl)pyrrolidine-2,5-dione
Abstract
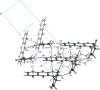
In the mol-ecular structure of title compound, C(18)H(13)NO(2), the succinimide ring is orientated away from the plane of the anthracene moiety by 71.94 (4)°. The crystal structure features three different types of inter-molecular inter-actions, viz. C-H⋯O, C-H⋯π and π-π bonds. Mol-ecules along the b axis stack on each other as a result of π-π inter-actions which have a centroid-centroid distance of 3.6780 (15) Å, while C-H⋯π inter-actions are present between neigbouring stacks. Also, acting between the stacks are the C-H⋯O inter-actions between the aromatic H atoms of the anthracene and the O atoms of the succinimide.
Figures
References
-
- Alston, P. V., Ottenbrite, R. M. & Newby, J. (1979). J. Org. Chem. 44, 4939–4943.
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
-
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
-
- Hubbard, J. L., Carl, J. M. III, Anderson, G. D. & Rankin, G. O. (1992). J. Heterocycl. Chem. 29, 719–721.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
