Strain-dependent differences in bone development, myeloid hyperplasia, morbidity and mortality in ptpn2-deficient mice
- PMID: 22590589
- PMCID: PMC3348136
- DOI: 10.1371/journal.pone.0036703
Strain-dependent differences in bone development, myeloid hyperplasia, morbidity and mortality in ptpn2-deficient mice
Abstract
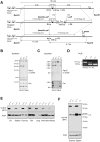
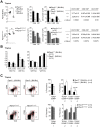
Single nucleotide polymorphisms in the gene encoding the protein tyrosine phosphatase TCPTP (encoded by PTPN2) have been linked with the development of autoimmunity. Here we have used Cre/LoxP recombination to generate Ptpn2(ex2-/ex2-) mice with a global deficiency in TCPTP on a C57BL/6 background and compared the phenotype of these mice to Ptpn2(-/-) mice (BALB/c-129SJ) generated previously by homologous recombination and backcrossed onto the BALB/c background. Ptpn2(ex2-/ex2-) mice exhibited growth retardation and a median survival of 32 days, as compared to 21 days for Ptpn2(-/-) (BALB/c) mice, but the overt signs of morbidity (hunched posture, piloerection, decreased mobility and diarrhoea) evident in Ptpn2(-/-) (BALB/c) mice were not detected in Ptpn2(ex2-/ex2-) mice. At 14 days of age, bone development was delayed in Ptpn2(-/-) (BALB/c) mice. This was associated with increased trabecular bone mass and decreased bone remodeling, a phenotype that was not evident in Ptpn2(ex2-/ex2-) mice. Ptpn2(ex2-/ex2-) mice had defects in erythropoiesis and B cell development as evident in Ptpn2(-/-) (BALB/c) mice, but not splenomegaly and did not exhibit an accumulation of myeloid cells in the spleen as seen in Ptpn2(-/-) (BALB/c) mice. Moreover, thymic atrophy, another feature of Ptpn2(-/-) (BALB/c) mice, was delayed in Ptpn2(ex2-/ex2-) mice and preceded by an increase in thymocyte positive selection and a concomitant increase in lymph node T cells. Backcrossing Ptpn2(-/-) (BALB/c) mice onto the C57BL/6 background largely recapitulated the phenotype of Ptpn2(ex2-/ex2-) mice. Taken together these results reaffirm TCPTP's important role in lymphocyte development and indicate that the effects on morbidity, mortality, bone development and the myeloid compartment are strain-dependent.
Conflict of interest statement
Figures


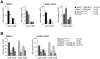
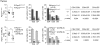


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

