A functional screen provides evidence for a conserved, regulatory, juxtamembrane phosphorylation site in guanylyl cyclase a and B
- PMID: 22590601
- PMCID: PMC3348905
- DOI: 10.1371/journal.pone.0036747
A functional screen provides evidence for a conserved, regulatory, juxtamembrane phosphorylation site in guanylyl cyclase a and B
Abstract
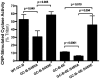
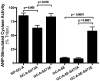
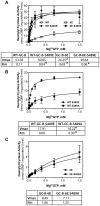
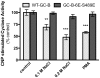
Kinase homology domain (KHD) phosphorylation is required for activation of guanylyl cyclase (GC)-A and -B. Phosphopeptide mapping identified multiple phosphorylation sites in GC-A and GC-B, but these approaches have difficulty identifying sites in poorly detected peptides. Here, a functional screen was conducted to identify novel sites. Conserved serines or threonines in the KHDs of phosphorylated receptor GCs were mutated to alanine and tested for reduced hormone to detergent activity ratios. Mutation of Ser-489 in GC-B to alanine but not glutamate reduced the activity ratio to 60% of wild type (WT) levels. Similar results were observed with Ser-473, the homologous site in GC-A. Receptors containing glutamates for previously identified phosphorylation sites (GC-A-6E and GC-B-6E) were activated to ~20% of WT levels but the additional glutamate substitution for S473 or S489 increased activity to near WT levels. Substrate-velocity assays indicated that GC-B-WT-S489E and GC-B-6E-S489E had lower Km values and that WT-GC-B-S489A, GC-B-6E and GC-B-6E-S489A had higher Km values than WT-GC-B. Homologous desensitization was enhanced when GC-A contained the S473E substitution, and GC-B-6E-S489E was resistant to inhibition by a calcium elevating treatment or protein kinase C activation--processes that dephosphorylate GC-B. Mass spectrometric detection of a synthetic phospho-Ser-473 containing peptide was 200-1300-fold less sensitive than other phosphorylated peptides and neither mass spectrometric nor (32)PO(4) co-migration studies detected phospho-Ser-473 or phospho-Ser-489 in cells. We conclude that Ser-473 and Ser-489 are Km-regulating phosphorylation sites that are difficult to detect using current methods.
Conflict of interest statement
Figures
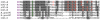



References
-
- Potter LR, Hunter T. Guanylyl cyclase-linked natriuretic peptide receptors: structure and regulation. The Journal of biological chemistry. 2001;276:6057–6060. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

