Soluble epoxide hydrolase activity determines the severity of ischemia-reperfusion injury in kidney
- PMID: 22590647
- PMCID: PMC3349654
- DOI: 10.1371/journal.pone.0037075
Soluble epoxide hydrolase activity determines the severity of ischemia-reperfusion injury in kidney
Abstract
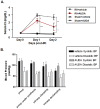
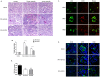
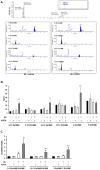
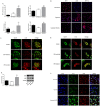
Soluble epoxide hydrolase (sEH) in endothelial cells determines the plasma concentrations of epoxyeicosatrienoic acids (EETs), which may act as vasoactive agents to control vascular tone. We hypothesized that the regulation of sEH activity may have a therapeutic value in preventing acute kidney injury by controlling the concentration of EETs. In this study, we therefore induced ischemia-reperfusion injury (IRI) in C57BL/6 mice and controlled sEH activity by intraperitoneal administration of the sEH inhibitor 12-(3-adamantan-1-ylureido)-dodecanoic acid (AUDA). The deterioration of kidney function induced by IRI was partially moderated and prevented by AUDA treatment. In addition, AUDA treatment significantly attenuated tubular necrosis induced by IRI. Ischemic injury induced the down-regulation of sEH, and AUDA administration had no effect on the expression pattern of sEH induced by IRI. In vivo sEH activity was assessed by measuring the substrate epoxyoctadecenoic acid (EpOME) and its metabolite dihydroxyoctadec-12-enoic acid (DHOME). Ischemic injury had no effects on the plasma concentrations of EpOME and DHOME, but inhibition of sEH by AUDA significantly increased plasma EpOME and the EpOME/DHOME ratio. The protective effect of the sEH inhibitor was achieved by suppression of proinflammatory cytokines and up-regulation of regulatory cytokines. AUDA treatment prevented the intrarenal infiltration of inflammatory cells, but promoted endothelial cell migration and neovascularization. The results of this study suggest that treatment with sEH inhibitors can reduce acute kidney injury.
Conflict of interest statement
Figures

References
-
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. - PubMed
-
- Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87:859–864. - PubMed
-
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

