The coronavirus E protein: assembly and beyond
- PMID: 22590676
- PMCID: PMC3347032
- DOI: 10.3390/v4030363
The coronavirus E protein: assembly and beyond
Abstract
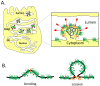
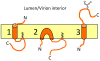
The coronavirus E protein is a small membrane protein that has an important role in the assembly of virions. Recent studies have indicated that the E protein has functions during infection beyond assembly, including in virus egress and in the host stress response. Additionally, the E protein has ion channel activity, interacts with host proteins, and may have multiple membrane topologies. The goal of this review is to highlight the properties and functions of the E protein, and speculate on how they may be related.
Keywords: Golgi complex; coronavirus assembly; envelope protein; ion channel; membrane protein topology.
Figures


References
-
- Hogue B.G., Machamer C.E. Coronavirus structural proteins and virus assembly. In: Perlman S., Gallagher T., Snijder E.J., editors. Nidoviruses. ASM Press; Washington, DC, USA: 2008. pp. 179–200.
-
- Boursnell M.E., Binns M.M., Brown T.D. Sequencing of coronavirus IBV genomic RNA: Three open reading frames in the 5' 'unique' region of mRNA D. J. Gen. Virol. 1985;66:2253–2258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

