Paramyxovirus fusion and entry: multiple paths to a common end
- PMID: 22590688
- PMCID: PMC3347325
- DOI: 10.3390/v4040613
Paramyxovirus fusion and entry: multiple paths to a common end
Abstract
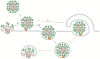
The paramyxovirus family contains many common human pathogenic viruses, including measles, mumps, the parainfluenza viruses, respiratory syncytial virus, human metapneumovirus, and the zoonotic henipaviruses, Hendra and Nipah. While the expression of a type 1 fusion protein and a type 2 attachment protein is common to all paramyxoviruses, there is considerable variation in viral attachment, the activation and triggering of the fusion protein, and the process of viral entry. In this review, we discuss recent advances in the understanding of paramyxovirus F protein-mediated membrane fusion, an essential process in viral infectivity. We also review the role of the other surface glycoproteins in receptor binding and viral entry, and the implications for viral infection. Throughout, we concentrate on the commonalities and differences in fusion triggering and viral entry among the members of the family. Finally, we highlight key unanswered questions and how further studies can identify novel targets for the development of therapeutic treatments against these human pathogens.
Keywords: membrane fusion; paramyxovirus; viral entry.
Figures


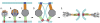
References
-
- Mebatsion T., Koolen M.J.M., de Vaan L.T.C., de Haas N., Braber M., Romer-Oberdorfer A., van den Elzen P., van der Marel P. Newcastle Disease Virus (NDV) marker vaccine: An immunodominant epitope on the nucleoprotein gene of NDV can be deleted or replaced by a foreign epitope. J. Virol. 2002;76:10138–10146. - PMC - PubMed
-
- Lamb R.A., Parks G.D. Paramyxoviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th. Vol. 1. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1449–1646.
-
- Chua K.B., Bellini W.J., Rota P.A., Harcourt B.H., Tamin A., Lam S.K., Ksiazek T.G., Rollin P.E., Zaki S.R., Shieh W., et al. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

