Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation
- PMID: 22591674
- PMCID: PMC3441391
- DOI: 10.1186/1479-5876-10-95
Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation
Abstract
Background: T cell activation is associated with a rapid increase in intracellular fructose-2,6-bisphosphate (F2,6BP), an allosteric activator of the glycolytic enzyme, 6-phosphofructo-1-kinase. The steady state concentration of F2,6BP in T cells is dependent on the expression of the bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFB1-4) and the fructose-2,6-bisphosphatase, TIGAR. Of the PFKFB family of enzymes, PFKFB3 has the highest kinase:bisphosphatase ratio and has been demonstrated to be required for T cell proliferation. A small molecule antagonist of PFKFB3, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO), recently has been shown to reduce F2,6BP synthesis, glucose uptake and proliferation in transformed cells. We hypothesized that the induction of PFKFB3 expression may be required for the stimulation of glycolysis in T cells and that exposure to the PFKFB3 antagonist, 3PO, would suppress T cell activation.
Methods: We examined PFKFB1-4 and TIGAR expression and F2,6BP concentration in purified CD3+ T cells stimulated with microbead-conjugated agonist antibodies specific for CD3 and the co-stimulatory receptor, CD28. We then determined the effect of 3PO on anti-CD3/anti-CD28-induced T cell activation, F2,6BP synthesis, 2-[1-14C]-deoxy-d-glucose uptake, lactate secretion, TNF-α secretion and proliferation. Finally, we examined the effect of 3PO administration on the development of delayed type hypersensitivity to methylated BSA and on imiquimod-induced psoriasis in mice.
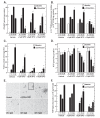
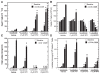
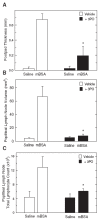
Results: We found that purified human CD3+ T cells express PFKFB2, PFKFB3, PFKFB4 and TIGAR, and that anti-CD3/anti-CD28 conjugated microbeads stimulated a >20-fold increase in F2,6BP with a coincident increase in protein expression of the PFKFB3 family member and a decrease in TIGAR protein expression. We then found that exposure to the PFKFB3 small molecule antagonist, 3PO (1-10 μM), markedly attenuated the stimulation of F2,6BP synthesis, 2-[1-14C]-deoxy-D-glucose uptake, lactate secretion, TNF-α secretion and T cell aggregation and proliferation. We examined the in vivo effect of 3PO on the development of delayed type hypersensitivity to methylated BSA and on imiquimod-induced psoriasis in mice and found that 3PO suppressed the development of both T cell-dependent models of immunity in vivo.
Conclusions: Our data demonstrate that inhibition of the PFKFB3 kinase activity attenuates the activation of T cells in vitro and suppresses T cell dependent immunity in vivo and indicate that small molecule antagonists of PFKFB3 may prove effective as T cell immunosuppressive agents.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

