Systems immunology of human malaria
- PMID: 22592005
- PMCID: PMC3361535
- DOI: 10.1016/j.pt.2012.03.006
Systems immunology of human malaria
Abstract
Plasmodium falciparum malaria remains a global public health threat. Optimism that a highly effective malaria vaccine can be developed stems in part from the observation that humans can acquire immunity to malaria through experimental and natural P. falciparum infection. Recent advances in systems immunology could accelerate efforts to unravel the mechanisms of acquired immunity to malaria. Here, we review the tools of systems immunology, their current limitations in the context of human malaria research, and the human 'models' of malaria immunity to which these tools can be applied.
Published by Elsevier Ltd.
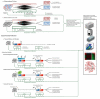
Figures
Similar articles
-
Advances and challenges in malaria vaccine development.J Clin Invest. 2010 Dec;120(12):4168-78. doi: 10.1172/JCI44423. Epub 2010 Dec 1. J Clin Invest. 2010. PMID: 21123952 Free PMC article. Review.
-
Overview: immunology of malaria and progress in malaria vaccine development.Southeast Asian J Trop Med Public Health. 1992 Sep;23 Suppl 4:71-87. Southeast Asian J Trop Med Public Health. 1992. PMID: 1364871 Review. No abstract available.
-
Genetically engineered parasites: the solution to designing an effective malaria vaccine?Trends Parasitol. 2010 Jul;26(7):322-3. doi: 10.1016/j.pt.2010.04.008. Epub 2010 May 25. Trends Parasitol. 2010. PMID: 20537954
-
Exploring immunological mechanisms of the whole sporozoite vaccination against P. falciparum malaria.Vaccine. 2015 Jun 9;33(25):2851-7. doi: 10.1016/j.vaccine.2015.04.056. Epub 2015 Apr 24. Vaccine. 2015. PMID: 25917675
-
Malaria Vaccines: Recent Advances and New Horizons.Cell Host Microbe. 2018 Jul 11;24(1):43-56. doi: 10.1016/j.chom.2018.06.008. Cell Host Microbe. 2018. PMID: 30001524 Free PMC article. Review.
Cited by
-
Humanized Mouse Models for the Study of Human Malaria Parasite Biology, Pathogenesis, and Immunity.Front Immunol. 2018 Apr 19;9:807. doi: 10.3389/fimmu.2018.00807. eCollection 2018. Front Immunol. 2018. PMID: 29725334 Free PMC article. Review.
-
HIV and co-infections.Immunol Rev. 2013 Jul;254(1):114-42. doi: 10.1111/imr.12063. Immunol Rev. 2013. PMID: 23772618 Free PMC article. Review.
-
Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease.Annu Rev Immunol. 2014;32:157-87. doi: 10.1146/annurev-immunol-032713-120220. Annu Rev Immunol. 2014. PMID: 24655294 Free PMC article. Review.
-
Young lives lost as B cells falter: what we are learning about antibody responses in malaria.J Immunol. 2013 Apr 1;190(7):3039-46. doi: 10.4049/jimmunol.1203067. J Immunol. 2013. PMID: 23526829 Free PMC article. Review.
-
CD4+ T cell response correlates with naturally acquired antibodies against Plasmodium vivax tryptophan-rich antigens.Infect Immun. 2015 May;83(5):2018-29. doi: 10.1128/IAI.03095-14. Epub 2015 Mar 2. Infect Immun. 2015. PMID: 25733522 Free PMC article.
References
-
- Murray CJ, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. - PubMed
-
- O’Meara WP, et al. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. - PubMed
-
- Ranson H, et al. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. - PubMed
-
- Agnandji ST, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

