Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents
- PMID: 22592307
- PMCID: PMC3424098
- DOI: 10.1152/jn.00094.2012
Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents
Abstract
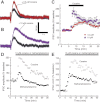
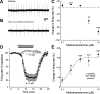
Amphetamine-like compounds are commonly used to enhance cognition and to treat attention deficit/hyperactivity disorder, but they also function as positive reinforcers and are self-administered at doses far exceeding clinical relevance. Many of these compounds (including methamphetamine) are substrates for dopamine reuptake transporters, elevating extracellular dopamine by inhibiting uptake and promoting reverse transport. This produces an increase in extracellular dopamine that inhibits dopamine neuron firing through autoreceptor activation and consequently blunts phasic dopamine neurotransmission, an important learning signal. However, these mechanisms do not explain the beneficial behavioral effects observed at clinically useful concentrations. In the present study, we have used patch-clamp electrophysiology in slices of mouse midbrain to show that, surprisingly, low concentrations of methamphetamine actually enhance dopamine neurotransmission and increase dopamine neuron firing through a dopamine transporter-mediated excitatory conductance. Both of these effects are reversed by higher concentrations of methamphetamine, which inhibit firing through dopamine D2 autoreceptor activation and decrease the peak amplitude of dopamine-mediated synaptic currents. These competing, concentration-dependent effects of methamphetamine suggest a mechanistic interplay by which lower concentrations of methamphetamine can overcome autoreceptor-mediated inhibition at the soma to increase phasic dopamine transmission.
Figures



References
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42: 939–946, 2004 - PubMed
-
- Brien JF, Kitney JC, Peachey JE, Rogers BJ. Methamphetamine-induced behavioural effects and brain concentrations of methamphetamine and its metabolite amphetamine in mice. Res Commun Chem Pathol Pharmacol 22: 313–328, 1978 - PubMed
-
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol 13: 569–574, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

