Highly fatal fast-channel syndrome caused by AChR ε subunit mutation at the agonist binding site
- PMID: 22592360
- PMCID: PMC3405251
- DOI: 10.1212/WNL.0b013e31825b5bda
Highly fatal fast-channel syndrome caused by AChR ε subunit mutation at the agonist binding site
Abstract
Objective: To characterize the molecular basis of a novel fast-channel congenital myasthenic syndrome.
Methods: We used the candidate gene approach to identify the pathogenic mutation in the acetylcholine receptor (AChR) ε subunit, genetically engineered the mutant AChR into HEK cells, and evaluated the level of expression and kinetic properties of the mutant receptor.
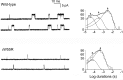
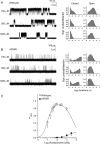
Results: An 8-year-old boy born to consanguineous parents had severe myasthenic symptoms since birth. He is wheelchair bound and pyridostigmine therapy enables him to take only a few steps. Three similarly affected siblings died in infancy. He carries a homozygous p.W55R mutation at the α/ε subunit interface of the AChR agonist binding site. The mutant protein expresses well in HEK cells. Patch-clamp analysis of the mutant receptor expressed in HEK cells reveals 30-fold reduced apparent agonist affinity, 75-fold reduced apparent gating efficiency, and strikingly attenuated channel opening probability (P(open)) over a range agonist concentrations.
Conclusion: Introduction of a cationic Arg into the anionic environment of α/ε AChR binding site hinders stabilization of cationic ACh by aromatic residues and accounts for the markedly perturbed kinetic properties of the receptor. The very low P(open) explains the poor response to pyridostigmine and the high fatality of the disease.
Figures
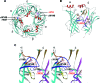
Comment in
-
Faster, slower, but never better: mutations of the skeletal muscle acetylcholine receptor.Neurology. 2012 Jul 31;79(5):404-5. doi: 10.1212/WNL.0b013e31825b5bee. Epub 2012 May 16. Neurology. 2012. PMID: 22592368 No abstract available.
References
-
- Engel AG. Congenital myasthenic syndromes. In: Aminoff MJ, ed. Handbook of Clinical Neurology Series: Disorders of Neuromuscular Transmission. New York: Elsevier; 2008: 285– 332 - PubMed
-
- Galzi JL, Revah F, Bessis A, Changeux JP. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol 1991; 31: 37– 72 - PubMed
-
- Sine SM. The nicotinic receptor ligand binding domain. J Neurobiol 2002; 53: 431– 446 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
