Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children
- PMID: 22592708
- PMCID: PMC7387678
- DOI: 10.1002/14651858.CD006100.pub2
Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children
Abstract
Background: Acute asthma presentation in the emergency setting frequently leads to hospital admission. Currently available treatment options include corticosteroid therapy, beta(2)-agonists and oxygen. Antileukotriene agents are beneficial in chronic asthma as additional therapy to inhaled steroids. Their value when used orally or intravenously in the acute setting requires evaluation.
Objectives: To determine if the addition of a leukotriene receptor antagonist (LTRA) produces a beneficial effect in children and adults with acute asthma who are currently receiving inhaled bronchodilators and systemic corticosteroids.
Search methods: We searched the Cochrane Airways Group's Specialised Register of trials with predefined terms. Searches are current to February 2012.
Selection criteria: We included randomised trials comparing antileukotrienes and standard acute asthma care versus placebo and standard care in people with acute asthma of any age. We considered any dose and method of delivery of the leukotriene agent.
Data collection and analysis: Two authors independently assessed studies for inclusion in the review and extracted data. We then checked data and resolved disagreements by discussion. We contacted study authors where necessary to provide additional information and data.
Main results: Eight trials, generating 10 treatment-control comparisons, that recruited 1470 adults and 470 children met the entry criteria. These studies were of mixed quality, and there was heterogeneity in the severity of asthma exacerbation.For oral treatment, there was no significant difference in hospital admission between LTRAs and control in three trials on 194 children (risk ratio (RR) 0.86; 95% confidence interval (CI) 0.21 to 3.52). Using a broader composite outcome which measured requirement for additional care there was no significant difference between treatments (RR 0.87; 95% CI 0.60 to 1.28). Results demonstrated some indication of improvement in lung function with a significant difference in forced expiratory volume in one second (FEV(1)) favouring LTRAs in two trials on 641 adults (mean difference (MD) 0.08; 95% CI 0.01 to 0.14). There were insufficient data to assess this outcome in children. The most common adverse event described was headache; however, there was no significant difference between LTRAs and control (RR 0.81; 95% CI 0.22 to 2.99). Due to insufficient numbers, we were unable to conduct a subgroup analysis based on age.The combined results of two trials of intravenous treatment in 772 adults and one trial in 276 children demonstrated a reduction in the risk of hospital admission which was not quite statistically significant (RR 0.78; 95% CI 0.61 to 1.01). There was a statistically significant small difference in FEV(1) in the adult studies (MD 0.12; 95% CI 0.06 to 0.17), but not in the single trial in children (MD 0.01; 95% CI -0.06 to 0.08).
Authors' conclusions: Presently, the available evidence does not support routine use of oral LTRAs in acute asthma. Further studies are required to assess whether intravenous treatment can reduce the risk of hospital admission, and what the most appropriate dose regimen is. Additional research is also needed into safety and efficacy of additional doses for those on maintenance therapy, and larger paediatric trials are required to allow subgroup analysis. Prolonged studies would be required to establish other health economic outcomes in admitted patients.
Conflict of interest statement
None known. The authors are neither involved with the makers of antileukotriene agents nor involved in the primary publications included in the review.
Figures
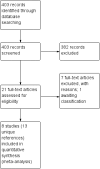
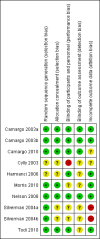





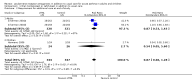













Update of
References
References to studies included in this review
Camargo 2003a {published data only}
-
- Camargo CA Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. American Journal of Respiratory and Critical Care Medicine 2003;167(4):528‐33. - PubMed
-
- Green SA, Camargo CA, Smithline HA, Nowak R, Malice MP, Reiss TF. Montelukast causes additive benefit in acute asthma. European Respiratory Journal 2001;18(Suppl 33):50s.
Camargo 2003b {published data only}
-
- Camargo CA Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. American Journal of Respiratory and Critical Care Medicine 2003;167(4):528‐33. - PubMed
-
- Green SA, Camargo CA, Smithline HA, Nowak R, Malice MP, Reiss TF. Montelukast causes additive benefit in acute asthma. European Respiratory Journal 2001;18(Suppl 33):50s.
Camargo 2010 {published data only}
-
- Camargo CA Jr, Smithline HA, Gurner DM, Malice MP, Legrand C, Green SA, et al. Evaluation of intravenous montelukast as an adjunctive treatment of acute asthma. American Journal of Respiratory and Critical Care Medicine. 2008; Vol. 177 (suppl):A71.
-
- Camargo Jr CA, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA, et al. A randomized placebo‐controlled study of intravenous montelukast for the treatment of acute asthma. Journal of Allergy and Clinical Immunology 2010; Vol. 125, issue 2:374‐80. - PubMed
Cylly 2003 {published data only}
-
- Cylly A, Kara A, Ozdemir T, Ogus C, Gulkesen KH. Effects of oral montelukast on airway function in acute asthma. Respiratory Medicine 2003;97(5):533‐6. - PubMed
Harmanci 2006 {published data only}
-
- Harmanci K, Bakirtas A, Turktas I, Degim T. Oral montelukast treatment of pre‐school‐aged children with acute asthma. Annals of allergy, asthma, and immunology 2006;96(5):731‐5. - PubMed
Morris 2010 {published data only}
-
- Morris CR, Becker AB, Pineiro A, Massaad R, Green SA, Smugar SS, et al. A randomized, placebo‐controlled study of intravenous montelukast in children with acute asthma. Annals of Allergy, Asthma and Immunology 2010; Vol. 104, issue 2:161‐71. - PubMed
Nelson 2008 {published data only}
-
- Nelson KA, Smith SR, Trinkaus K, Jaffe DM. Pilot study of oral montelukast added to standard therapy for acute asthma exacerbations in children aged 6 to 14 years. Pediatric Emergency Care 2008;24(1):21‐7. - PubMed
Silverman 2004a {published data only}
-
- Anonymous. High‐dose zafirlukast in emergency department provides small benefit in acute asthma. The Journal of Family Practice 2005;54(4):304‐6. - PubMed
-
- Korenblatt PE, Silverman RA, Nowak RM, Chen Y, Bonuccelli CM, Miller CJ, et al. Zafirlukast improves outpatient outcomes after acute asthma treatment. Annals of Allergy, Asthma & Immunology 2000;84(1):18.
-
- Silverman RA, Nowak RM, Korenblat PE, Skobeloff E, Chen Y, Bonuccelli CM, et al. Zafirlukast treatment for acute asthma: evaluation in a randomized, double‐blind, multi‐center trial. Chest 2004;126(5):1480‐9. - PubMed
-
- Silvermann RA, Korenblat PE, Nowak RM, Chen Y, Bonnuccelli CM, Miller CJ, et al. Zafirlukast improves emergency department outcomes and reduces relapses after an acute asthma episode. European Respiratory Journal 2000;16(Supplement 31):524s.
Silverman 2004b {published data only}
-
- Anonymous. High‐dose zafirlukast in emergency department provides small benefit in acute asthma. The Journal of Family Practice 2005;54(4):304‐6. - PubMed
-
- Korenblatt PE, Silverman RA, Nowak RM, Chen Y, Bonuccelli CM, Miller CJ, et al. Zafirlukast improves outpatient outcomes after acute asthma treatment. Annals of Allergy, Asthma and Immunology 2000;84(1):18.
-
- Silverman RA, Nowak RM, Korenblat PE, Skobeloff E, Chen Y, Bonuccelli CM, et al. Zafirlukast treatment for acute asthma: evaluation in a randomized, double‐blind, multi‐center trial. Chest 2004;126(5):1480‐9. - PubMed
-
- Silvermann RA, Korenblat PE, Nowak RM, Chen Y, Bonnuccelli CM, Miller CJ, et al. Zafirlukast improves emergency department outcomes and reduces relapses after an acute asthma episode. European Respiratory Journal 2000;16(Suppl 31):524s.
Todi 2010 {published data only}
-
- Todi VK, Lodha R, Kabra SK. Effect of addition of single dose of oral montelukast to standard treatment in acute moderate to severe asthma in children between 5 and 15 years of age: A randomised, double‐blind, placebo controlled trial. Archives of Disease in Childhood 2010; Vol. 95, issue 7:540‐3. - PubMed
References to studies excluded from this review
Bacharier 2008 {published data only}
-
- Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate‐to‐severe intermittent wheezing. The Journal of allergy and clinical immunology 2008; Vol. 122, issue 6:1127‐35. - PMC - PubMed
Gulati 2005 {published data only}
-
- Gulati A, Kabwa S, Jacob BK. A randomised controlled trial of zafirlukast in acute asthma. European Respiratory Journal 2005;26(Suppl 49):254s.
Matsunga 2004 {published data only}
-
- Matsunga K, Nishimoto T, Hirano T, Nakanishi M, Yamagata T, Kuroda M, et al. Effect of a leukotriene receptor antagonist on the prevention of recurrent asthma attacks after an emergency room visit. Allergology International 2004;53(4):341‐7.
Ramsay 2007 {published data only}
-
- Ramsay C, Pearson D, Wilson A. Oral montelukast in patients with acute asthma. European Respiratory Society, 17th European Respiratory Society Annual Congress, Stockholm, Sweden, September 14‐19, 2007. 2007; Vol. 30 (suppl 51):615s [P3609].
Robertson 2007 {published data only}
-
- Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, et al. Short‐course montelukast for intermittent asthma in children: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2007; Vol. 175, issue 4:323‐9. [CN‐00576949] - PubMed
Schuh 2009 {published data only}
-
- Schuh S, Willan AR, Stephens D, Dick PT, Coates A. Can montelukast shorten prednisolone therapy in children with mild to moderate acute asthma? A randomized controlled trial. Journal of Pediatrics 2009; Vol. 155, issue 6:795‐800. - PubMed
Zubairi 2007 {published data only}
-
- Zubairi ABS, Haque AS, Waheed S, Awan S. A randomized, double‐blinded, placebo‐controlled clinical trial of oral montelukast in acute asthma exacerbation (PTOMA trial). Respirology, 13th Congress of the Asian Pacific. Society of Respirology: Optimal Use of Advanced Technology, 19‐22 November, Bangkok, Thailand. 2008; Vol. 13:A131 [018‐01].
References to studies awaiting assessment
Adachi 2008 {published data only}
-
- Adachi M, Sano Y, Tohda Y, Taniguchi H, Fujisawa T, Hiraga S, et al. Efficacy and safety of intravenous montelukast in patients with acute exacerbations of bronchial asthma. European Respiratory Society. 2008.
Additional references
Blitz 2005
BTS/SIGN
-
- British Guideline on the Management of Asthma; a national clinical guideline. http://www.brit‐thoracic.org.uk/guidelines/asthma‐guidelines.aspx 2008, revised 2011 (accessed 2011).
Dockhorn 2000
Ducharme 2004
GINA 2008
-
- Agostinis F, Foglia C, Landi M, Cottini M, Lombardi C, Canonica GW, et al. GINA Report: global strategy for asthma management and prevention. Allergy 2008;63(12):1637‐9. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mitra 2005
NAEPP 2007
-
- National Asthma Education and Prevention Program, National Heart, Blood and Lung Institute. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm 2007 (accessed 2011).
Parameswaran 2000
Plotnick 2000
Review Manager 5 [Computer program]
-
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Rowe 2000
Sampson 1995
Stoodley 1999
-
- Stoodley RG, Aaron SD, Dales RE. The role of ipratropium bromide in the emergency management of acute asthma exacerbation: a meta‐analysis of randomized clinical trials. Annals of Emergency Medicine 1999;34(1):8‐18. - PubMed
Travers 2001
Wu 2003
-
- Wu AY, Chik SC, Chan AW, Li Z, Tsang KW, Li W. Anti‐inflammatory effects of high‐dose montelukast in an animal model of acute asthma. Clinical Experimental Allergy 2003;33(3):359‐66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

