Radiotherapy and chemoradiation after surgery for early cervical cancer
- PMID: 22592722
- PMCID: PMC4171000
- DOI: 10.1002/14651858.CD007583.pub3
Radiotherapy and chemoradiation after surgery for early cervical cancer
Abstract
Background: This is an updated version of the original Cochrane review first published in Issue 4, 2009. There is an ongoing debate about the indications for, and value of, adjuvant pelvic radiotherapy after radical surgery in women with early cervical cancer. Certain combinations of pathological risk factors are thought to represent sufficient risk for recurrence, that they justify the use of postoperative pelvic radiotherapy, though this has never been shown to improve overall survival, and use of more than one type of treatment (surgery and radiotherapy) increases the risks of side effects and complications.
Objectives: To evaluate the effectiveness and safety of adjuvant therapies (radiotherapy, chemotherapy followed by radiotherapy, chemoradiation) after radical hysterectomy for early-stage cervical cancer (FIGO stages IB1, IB2 or IIA).
Search methods: For the original review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 4, 2008. The Cochrane Gynaecological Cancer Group Trials Register, MEDLINE (January 1950 to November 2008), EMBASE (1950 to November 2008). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field. For this update, we extended the database searches to September 2011 and searched the MetaRegister for ongoing trials.
Selection criteria: Randomised controlled trials (RCTs) that compared adjuvant therapies (radiotherapy, chemotherapy followed by radiotherapy, or chemoradiation) with no radiotherapy or chemoradiation, in women with a confirmed histological diagnosis of early cervical cancer who had undergone radical hysterectomy and dissection of the pelvic lymph nodes.
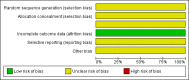
Data collection and analysis: Two review authors independently abstracted data and assessed risk of bias. Information on grade 3 and 4 adverse events was collected from the trials. Results were pooled using random-effects meta-analyses.
Main results: Two RCTs, which compared adjuvant radiotherapy with no adjuvant radiotherapy, met the inclusion criteria; they randomised and assessed 397 women with stage IB cervical cancer. Meta-analysis of these two RCTs indicated no significant difference in survival at 5 years between women who received radiation and those who received no further treatment (risk ratio (RR) = 0.8; 95% confidence interval (CI) 0.3 to 2.4). However, women who received radiation had a significantly lower risk of disease progression at 5 years (RR 0.6; 95% CI 0.4 to 0.9).Although the risk of serious adverse events was consistently higher if women received radiotherapy rather than no further treatment, these increased risks were not statistically significant, probably because the rate of adverse events was low.
Authors' conclusions: We found evidence, of moderate quality, that radiation decreases the risk of disease progression compared with no further treatment, but little evidence that it might improve overall survival, in stage IB cervical cancer. The evidence on serious adverse events was equivocal.
Conflict of interest statement
None.
Figures



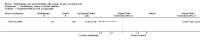









Update of
-
Adjuvant radiotherapy and chemoradiation after surgery for cervical cancer.Cochrane Database Syst Rev. 2009 Oct 7;(4):CD007583. doi: 10.1002/14651858.CD007583.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 May 16;(5):CD007583. doi: 10.1002/14651858.CD007583.pub3. PMID: 19821430 Updated.
References
References to studies included in this review
Bilek 1982 {published data only}
-
- Bilek K, Ebeling K, Leitsmann H, Seidel G. Radical pelvic surgery versus radical surgery plus radiotherapy for stage IB carcinoma of the cervix uteri: preliminary results of a prospective randomised clinical study. Archiv fur Geschwulstforschung 1982;52(3):223‐29. - PubMed
GOG 92 2006 {published data only}
-
- Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow‐up of a gynaecologic oncology group study. International Journal of Radiation Oncology and Biological Physics 2006;65(1):169–76. - PubMed
-
- Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study. Gynecologic Oncology 1999;73:177‐83. - PubMed
References to studies excluded from this review
Lahousen 1999 {published data only}
-
- Lahousen M, Haas J, Pickel H, Hackl A, Kurz C, Ogris H, et al. Chemotherapy versus radiotherapy versus observation for high‐risk cervical carcinoma after radical hysterectomy: a randomized, prospective, multicenter trial. Gynecologic Oncology 1999;73:196–201. - PubMed
Additional references
Benedet 2000
-
- Benedet JL, Hacker NF, Ngan HYS. Staging classifications and clinical practice guidelines of gynaecologic cancers by FIGO Committee on Gynecologic Oncology and IGCS Guidelines Committee. International Journal of Gynaecology and Obstetrics 2000;70:207‐312. - PubMed
CCCMAC 2010
-
- Vale C, Tierney JF, Stewart LA, Brady M, Dinshaw K, Jakobsen A, et al (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta‐analysis (Review). Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008285] - DOI - PMC - PubMed
CTCAE 2006
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.h... (accessed 15 March 2012).
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Egger M, Davey Smith G, Altman DG (editors). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group, 2001:313‐35.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
EUROCARE 2003
-
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al and the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14(Suppl 5):v61‐v118. - PubMed
GLOBOCAN 2008
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008. Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10, version 2.0. IARC Press, Lyon, France, 2010. Available from http://globocan.iarc.fr.
Gray 2008
-
- Gray HJ. Primary management of early stage cervical cancer (IA1‐IB) and appropriate selection of adjuvant therapy. Journal of the National Comprehensive Cancer Network 2008;6(1):47‐52. - PubMed
Guttmann 1970
-
- Guttmann R. Significance of post‐operative irradiation in carcinoma of the cervix: a ten year survey. American Journal of Roentgenology 1970;108:102‐8. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kesic 2006
-
- Kesic V. Management of cervical cancer. European Journal of Surgical Oncology 2006;32(8):832‐7. - PubMed
Kitchener 2010
-
- Kitchener HC, Hoskins W, Small, W, Thomas GM, Trimble EL and the Cervical Cancer Consensus Group. The development of priority cervical cancer trials: a Gynecologic Cancer InterGroup report. International Journal of Gynecological Cancer 2010;20:1092‐1100. - PubMed
Moher 1998
-
- Moher D, Pham D, Jones A, Cook DJ, Jadad AR, Moher M. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Peters 2000
-
- Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. Journal of Clinical Oncology 2000;18(8):1606‐13. - PubMed
Rosa 2009
Rydzewska 2010
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Sedlis 1999
-
- Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage Ib carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecologic Oncology 1999;73(2):177‐83. - PubMed

