Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens
- PMID: 22593064
- PMCID: PMC3360066
- DOI: 10.1523/JNEUROSCI.5718-11.2012
Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens
Abstract
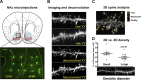
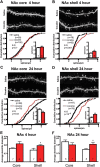
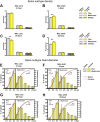
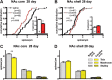
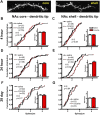
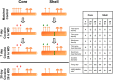
Numerous studies have found that chronic cocaine increases dendritic spine density of medium spiny neurons in the nucleus accumbens (NAc). Here, we used single-cell microinjections and advanced 3D imaging and analysis techniques to extend these findings in several important ways: by assessing cocaine regulation of dendritic spines in the core versus shell subregions of NAc in the mouse, over a broad time course (4 h, 24 h, or 28 d) of withdrawal from chronic cocaine, and with a particular focus on proximal versus distal dendrites. Our data demonstrate subregion-specific, and in some cases opposite, regulation of spines by cocaine on proximal but not distal dendrites. Notably, all observed density changes were attributable to selective regulation of thin spines. At 4 h after injection, the proximal spine density is unchanged in the core but significantly increased in the shell. At 24 h, the density of proximal dendritic spines is reduced in the core but increased in the shell. Such downregulation of thin spines in the core persists through 28 d of withdrawal, whereas the spine density in the shell returns to baseline levels. Consistent with previous results, dendritic tips exhibited upregulation of dendritic spines after 24 h of withdrawal, an effect localized to the shell. The divergence in regulation of proximal spine density in NAc core versus shell by cocaine correlates with recently reported electrophysiological data from a similar drug administration regimen and might represent a key mediator of changes in the reward circuit that drive aspects of addiction.
Figures
References
-
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. - PubMed
-
- Branco T, Häusser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 2010;20:494–502. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
