S-SCAM/MAGI-2 is an essential synaptic scaffolding molecule for the GluA2-containing maintenance pool of AMPA receptors
- PMID: 22593065
- PMCID: PMC3365591
- DOI: 10.1523/JNEUROSCI.0025-12.2012
S-SCAM/MAGI-2 is an essential synaptic scaffolding molecule for the GluA2-containing maintenance pool of AMPA receptors
Abstract
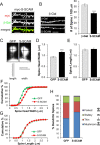
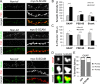
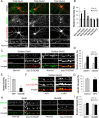
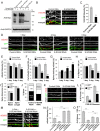
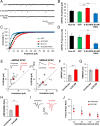
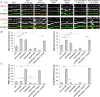
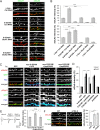
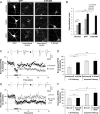
Synaptic plasticity, the cellular basis of learning and memory, involves the dynamic trafficking of AMPA receptors (AMPARs) into and out of synapses. One of the remaining key unanswered aspects of AMPAR trafficking is the mechanism by which synaptic strength is preserved despite protein turnover. In particular, the identity of AMPAR scaffolding molecule(s) involved in the maintenance of GluA2-containing AMPARs is completely unknown. Here we report that the synaptic scaffolding molecule (S-SCAM; also called membrane-associated guanylate kinase inverted-2 and atrophin interacting protein-1) plays the critical role of maintaining synaptic strength. Increasing S-SCAM levels in rat hippocampal neurons led to specific increases in the surface AMPAR levels, enhanced AMPAR-mediated synaptic transmission, and enlargement of dendritic spines, without significantly effecting GluN levels or NMDA receptor (NMDAR) EPSC. Conversely, decreasing S-SCAM levels by RNA interference-mediated knockdown caused the loss of synaptic AMPARs, which was followed by a severe reduction in the dendritic spine density. Importantly, S-SCAM regulated synaptic AMPAR levels in a manner, dependent on GluA2 not GluA1, sensitive to N-ethylmaleimide-sensitive fusion protein interaction, and independent of activity. Further, S-SCAM increased surface AMPAR levels in the absence of PSD-95, while PSD-95 was dependent on S-SCAM to increase surface AMPAR levels. Finally, S-SCAM overexpression hampered NMDA-induced internalization of AMPARs and prevented the induction of long term-depression, while S-SCAM knockdown did not. Together, these results suggest that S-SCAM is an essential AMPAR scaffolding molecule for the GluA2-containing pool of AMPARs, which are involved in the constitutive pathway of maintaining synaptic strength.
Figures
References
-
- Buxbaum JD, Georgieva L, Young JJ, Plescia C, Kajiwara Y, Jiang Y, Moskvina V, Norton N, Peirce T, Williams H, Craddock NJ, Carroll L, Corfas G, Davis KL, Owen MJ, Harroch S, Sakurai T, O'Donovan MC. Molecular dissection of NRG1-ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. Mol Psychiatry. 2008;13:162–172. - PMC - PubMed
-
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
