Regulation of Fasciclin II and synaptic terminal development by the splicing factor beag
- PMID: 22593074
- PMCID: PMC3375121
- DOI: 10.1523/JNEUROSCI.3717-11.2012
Regulation of Fasciclin II and synaptic terminal development by the splicing factor beag
Abstract
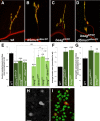
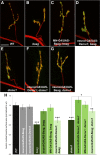
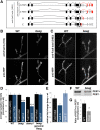
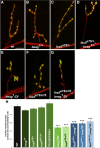
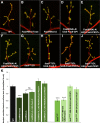
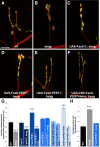
Pre-mRNA alternative splicing is an important mechanism for the generation of synaptic protein diversity, but few factors governing this process have been identified. From a screen for Drosophila mutants with aberrant synaptic development, we identified beag, a mutant with fewer synaptic boutons and decreased neurotransmitter release. Beag encodes a spliceosomal protein similar to splicing factors in humans and Caenorhabditis elegans. We find that both beag mutants and mutants of an interacting gene dsmu1 have changes in the synaptic levels of specific splice isoforms of Fasciclin II (FasII), the Drosophila ortholog of neural cell adhesion molecule. We show that restoration of one splice isoform of FasII can rescue synaptic morphology in beag mutants while expression of other isoforms cannot. We further demonstrate that this FasII isoform has unique functions in synaptic development independent of transsynaptic adhesion. beag and dsmu1 mutants demonstrate an essential role for these previously uncharacterized splicing factors in the regulation of synapse development and function.
Figures


References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Assier E, Bouzinba-Segard H, Stolzenberg MC, Stephens R, Bardos J, Freemont P, Charron D, Trowsdale J, Rich T. Isolation, sequencing and expression of RED, a novel human gene encoding an acidic-basic dipeptide repeat. Gene. 1999;230:145–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
