CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients
- PMID: 22593175
- PMCID: PMC4425934
- DOI: 10.1126/scitranslmed.3003330
CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients
Abstract
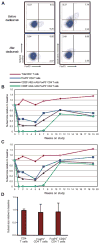
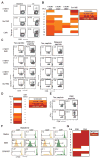
Regulatory T cells (T(regs)) are key mediators of immune tolerance and feature prominently in cancer. Depletion of CD25(+) FoxP3(+) T(regs) in vivo may promote T cell cancer immunosurveillance, but no strategy to do so in humans while preserving immunity and preventing autoimmunity has been validated. We evaluated the Food and Drug Administration-approved CD25-blocking monoclonal antibody daclizumab with regard to human T(reg) survival and function. In vitro, daclizumab did not mediate antibody-dependent or complement-mediated cytotoxicity but rather resulted in the down-regulation of FoxP3 selectively among CD25(high) CD45RA(neg) T(regs). Moreover, daclizumab-treated CD45RA(neg) T(regs) lost suppressive function and regained the ability to produce interferon-γ, consistent with reprogramming. To understand the impact of daclizumab on T(regs) in vivo, we performed a clinical trial of daclizumab in combination with an experimental cancer vaccine in patients with metastatic breast cancer. Daclizumab administration led to a marked and prolonged decrease in T(regs) in patients. Robust CD8 and CD4 T cell priming and boosting to all vaccine antigens were observed in the absence of autoimmunity. We conclude that CD25 blockade depletes and selectively reprograms T(regs) in concert with active immune therapy in cancer patients. These results suggest a mechanism to target cancer-associated T(regs) while avoiding autoimmunity.
Conflict of interest statement
Figures


Comment in
-
Depletion of Tregs in vivo: a promising approach to enhance antitumor immunity without autoimmunity.Immunotherapy. 2012 Nov;4(11):1103-5. doi: 10.2217/imt.12.116. Immunotherapy. 2012. PMID: 23194360
References
-
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. - PubMed
-
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

