A local paracrine and endocrine network involving TGFβ, Cox-2, ROS, and estrogen receptor β influences reactive stromal cell regulation of prostate cancer cell motility
- PMID: 22593181
- PMCID: PMC3355541
- DOI: 10.1210/me.2011-1371
A local paracrine and endocrine network involving TGFβ, Cox-2, ROS, and estrogen receptor β influences reactive stromal cell regulation of prostate cancer cell motility
Erratum in
- Mol Endocrinol. 2014 Feb;28(2):275
Abstract
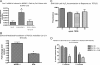
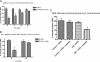
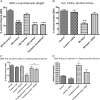
The tumor microenvironment plays a critical role in supporting cancer cells particularly as they disengage from limitations on their growth and motility imposed by surrounding nonreactive stromal cells. We show here that stromal-derived androgenic precursors are metabolized by DU145 human prostate cancer (PCa) cells to generate ligands for estrogen receptor-β, which act to limit their motility through transcriptional regulation of E-cadherin. Although primary human PCa-associated fibroblasts and the human WPMY-1-reactive prostate stromal cell line maintain this inherent estrogen receptor (ER)β-dependent motility inhibitor activity, they are subverted by TGF-β1 pro-oxidant signals derived from cocultured DU145 PCa cells. Specifically, stromal-produced H(2)O(2), which requires Cox-2, acts as a second paracrine factor to inhibit ERβ activity in adjacent DU145 cells. Chromatin immunoprecipitation analysis reveals that ERβ recruitment to the E-cadherin promoter is inhibited when H(2)O(2) is present. Both neutralization of H(2)O(2) with catalase and prevention of its production by silencing Cox-2 expression in stromal cells restore the motility-suppression activity of stromal-derived ERβ ligand precursors. These data suggest that reactive stromal cells may still have a capacity to limit cancer cell motility through a local endocrine network but must be protected from pro-oxidant signals triggered by cancer cell-derived TGF-β1 to exhibit this cancer-suppressive function.
Figures





References
-
- Paget S. 1989. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101 - PubMed
-
- Cunha GR. 1994. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 74:1030–1044 - PubMed
-
- Cunha GR, Hayward SW, Wang YZ. 2002. Role of stroma in carcinogenesis of the prostate. Differentiation 70:473–485 - PubMed
-
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. 2002. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8:2912–2923 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

