Overexpression of the Coq8 kinase in Saccharomyces cerevisiae coq null mutants allows for accumulation of diagnostic intermediates of the coenzyme Q6 biosynthetic pathway
- PMID: 22593570
- PMCID: PMC3390632
- DOI: 10.1074/jbc.M112.360354
Overexpression of the Coq8 kinase in Saccharomyces cerevisiae coq null mutants allows for accumulation of diagnostic intermediates of the coenzyme Q6 biosynthetic pathway
Abstract
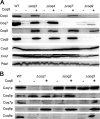
Most of the Coq proteins involved in coenzyme Q (ubiquinone or Q) biosynthesis are interdependent within a multiprotein complex in the yeast Saccharomyces cerevisiae. Lack of only one Coq polypeptide, as in Δcoq strains, results in the degradation of several Coq proteins. Consequently, Δcoq strains accumulate the same early intermediate of the Q(6) biosynthetic pathway; this intermediate is therefore not informative about the deficient biosynthetic step in a particular Δcoq strain. In this work, we report that the overexpression of the protein Coq8 in Δcoq strains restores steady state levels of the unstable Coq proteins. Coq8 has been proposed to be a kinase, and we provide evidence that the kinase activity is essential for the stabilizing effect of Coq8 in the Δcoq strains. This stabilization results in the accumulation of several novel Q(6) biosynthetic intermediates. These Q intermediates identify chemical steps impaired in cells lacking Coq4 and Coq9 polypeptides, for which no function has been established to date. Several of the new intermediates contain a C4-amine and provide information on the deamination reaction that takes place when para-aminobenzoic acid is used as a ring precursor of Q(6). Finally, we used synthetic analogues of 4-hydroxybenzoic acid to bypass deficient biosynthetic steps, and we show here that 2,4-dihydroxybenzoic acid is able to restore Q(6) biosynthesis and respiratory growth in a Δcoq7 strain overexpressing Coq8. The overexpression of Coq8 and the use of 4-hydroxybenzoic acid analogues represent innovative tools to elucidate the Q biosynthetic pathway.
Figures






Similar articles
-
Coenzyme Q supplementation or over-expression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants.Biochim Biophys Acta. 2014 Apr 4;1841(4):630-44. doi: 10.1016/j.bbalip.2013.12.017. Epub 2014 Jan 7. Biochim Biophys Acta. 2014. PMID: 24406904 Free PMC article.
-
Yeast Coq9 controls deamination of coenzyme Q intermediates that derive from para-aminobenzoic acid.Biochim Biophys Acta. 2015 Sep;1851(9):1227-39. doi: 10.1016/j.bbalip.2015.05.003. Epub 2015 May 23. Biochim Biophys Acta. 2015. PMID: 26008578 Free PMC article.
-
Expression of the human atypical kinase ADCK3 rescues coenzyme Q biosynthesis and phosphorylation of Coq polypeptides in yeast coq8 mutants.Biochim Biophys Acta. 2011 May;1811(5):348-60. doi: 10.1016/j.bbalip.2011.01.009. Epub 2011 Feb 4. Biochim Biophys Acta. 2011. PMID: 21296186 Free PMC article.
-
Coenzyme Q10 deficiencies: pathways in yeast and humans.Essays Biochem. 2018 Jul 20;62(3):361-376. doi: 10.1042/EBC20170106. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 29980630 Free PMC article. Review.
-
Regulation of coenzyme Q biosynthesis in yeast: a new complex in the block.IUBMB Life. 2014 Feb;66(2):63-70. doi: 10.1002/iub.1243. Epub 2014 Jan 27. IUBMB Life. 2014. PMID: 24470391 Review.
Cited by
-
Mitochondrial dysfunction, aging, and the mitochondrial unfolded protein response in Caenorhabditis elegans.Genetics. 2022 Nov 30;222(4):iyac160. doi: 10.1093/genetics/iyac160. Genetics. 2022. PMID: 36342845 Free PMC article. Review.
-
Resveratrol and para-coumarate serve as ring precursors for coenzyme Q biosynthesis.J Lipid Res. 2015 Apr;56(4):909-19. doi: 10.1194/jlr.M057919. Epub 2015 Feb 14. J Lipid Res. 2015. PMID: 25681964 Free PMC article.
-
Impact of Chemical Analogs of 4-Hydroxybenzoic Acid on Coenzyme Q Biosynthesis: From Inhibition to Bypass of Coenzyme Q Deficiency.Front Physiol. 2017 Jun 22;8:436. doi: 10.3389/fphys.2017.00436. eCollection 2017. Front Physiol. 2017. PMID: 28690551 Free PMC article. Review.
-
Human COQ9 Rescues a coq9 Yeast Mutant by Enhancing Coenzyme Q Biosynthesis from 4-Hydroxybenzoic Acid and Stabilizing the CoQ-Synthome.Front Physiol. 2017 Jul 7;8:463. doi: 10.3389/fphys.2017.00463. eCollection 2017. Front Physiol. 2017. PMID: 28736527 Free PMC article.
-
A conserved START domain coenzyme Q-binding polypeptide is required for efficient Q biosynthesis, respiratory electron transport, and antioxidant function in Saccharomyces cerevisiae.Biochim Biophys Acta. 2013 Apr;1831(4):776-791. doi: 10.1016/j.bbalip.2012.12.007. Epub 2012 Dec 25. Biochim Biophys Acta. 2013. PMID: 23270816 Free PMC article.
References
-
- Bentinger M., Tekle M., Dallner G. (2010) Coenzyme Q-biosynthesis and functions. Biochem. Biophys. Res. Commun. 396, 74–79 - PubMed
-
- Pierrel F., Hamelin O., Douki T., Kieffer-Jaquinod S., Mühlenhoff U., Ozeir M., Lill R., Fontecave M. (2010) Involvement of mitochondrial ferredoxin and para-aminobenzoic acid in yeast coenzyme Q biosynthesis. Chem. Biol. 17, 449–459 - PubMed
-
- Marbois B., Gin P., Faull K. F., Poon W. W., Lee P. T., Strahan J., Shepherd J. N., Clarke C. F. (2005) Coq3 and Coq4 define a polypeptide complex in yeast mitochondria for the biosynthesis of coenzyme Q. J. Biol. Chem. 280, 20231–20238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

