Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine
- PMID: 22593577
- PMCID: PMC3391103
- DOI: 10.1074/jbc.M112.372029
Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine
Abstract
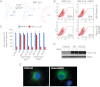
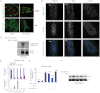
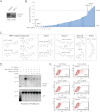
Secreted Wnt proteins constitute one of the largest families of intercellular signaling molecules in vertebrates with essential roles in embryonic development and adult tissue homeostasis. The functional redundancy of Wnt genes and the many forms of cellular responses they elicit, including some utilizing the transcriptional co-activator β-catenin, has limited the ability of classical genetic strategies to uncover their roles in vivo. We had previously identified a chemical compound class termed Inhibitor of Wnt Production (or IWP) that targets Porcupine (Porcn), an acyltransferase catalyzing the addition of fatty acid adducts onto Wnt proteins. Here we demonstrate that diverse chemical structures are able to inhibit Porcn by targeting its putative active site. When deployed in concert with small molecules that modulate the activity of Tankyrase enzymes and glycogen synthase kinase 3 β (GSK3β), additional transducers of Wnt/β-catenin signaling, the IWP compounds reveal an essential role for Wnt protein fatty acylation in eliciting β-catenin-dependent and -independent forms of Wnt signaling during zebrafish development. This collection of small molecules facilitates rapid dissection of Wnt gene function in vivo by limiting the influence of redundant Wnt gene functions on phenotypic outcomes and enables temporal manipulation of Wnt-mediated signaling in vertebrates.
Figures


Similar articles
-
Stereoselective fatty acylation is essential for the release of lipidated WNT proteins from the acyltransferase Porcupine (PORCN).J Biol Chem. 2019 Apr 19;294(16):6273-6282. doi: 10.1074/jbc.RA118.007268. Epub 2019 Feb 8. J Biol Chem. 2019. PMID: 30737280 Free PMC article.
-
An in vitro fatty acylation assay reveals a mechanism for Wnt recognition by the acyltransferase Porcupine.J Biol Chem. 2017 Aug 18;292(33):13507-13513. doi: 10.1074/jbc.C117.800136. Epub 2017 Jun 27. J Biol Chem. 2017. PMID: 28655768 Free PMC article.
-
Porcupine inhibitors: Novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway.Pharmacol Res. 2021 May;167:105532. doi: 10.1016/j.phrs.2021.105532. Epub 2021 Mar 4. Pharmacol Res. 2021. PMID: 33677106 Review.
-
Identification of key residues and regions important for porcupine-mediated Wnt acylation.J Biol Chem. 2014 Jun 13;289(24):17009-19. doi: 10.1074/jbc.M114.561209. Epub 2014 May 5. J Biol Chem. 2014. PMID: 24798332 Free PMC article.
-
Modulating Wnt signaling at the root: Porcupine and Wnt acylation.Pharmacol Ther. 2019 Jun;198:34-45. doi: 10.1016/j.pharmthera.2019.02.009. Epub 2019 Feb 18. Pharmacol Ther. 2019. PMID: 30790642 Review.
Cited by
-
Wnt5A Signaling Regulates Gut Bacterial Survival and T Cell Homeostasis.mSphere. 2022 Dec 21;7(6):e0050722. doi: 10.1128/msphere.00507-22. Epub 2022 Dec 6. mSphere. 2022. PMID: 36472447 Free PMC article.
-
Fatty acylation of Wnt proteins.Nat Chem Biol. 2016 Feb;12(2):60-9. doi: 10.1038/nchembio.2005. Nat Chem Biol. 2016. PMID: 26784846
-
Development of a triazole class of highly potent Porcn inhibitors.Bioorg Med Chem Lett. 2016 Dec 15;26(24):5891-5895. doi: 10.1016/j.bmcl.2016.11.012. Epub 2016 Nov 11. Bioorg Med Chem Lett. 2016. PMID: 27876319 Free PMC article.
-
Divergent effects of Porcupine and Wntless on WNT1 trafficking, secretion, and signaling.Exp Cell Res. 2016 Sep 10;347(1):171-183. doi: 10.1016/j.yexcr.2016.07.028. Epub 2016 Aug 1. Exp Cell Res. 2016. PMID: 27492485 Free PMC article.
-
Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins.Cell Rep. 2013 Sep 26;4(6):1072-81. doi: 10.1016/j.celrep.2013.08.027. Epub 2013 Sep 19. Cell Rep. 2013. PMID: 24055053 Free PMC article.
References
-
- Angers S., Moon R. T. (2009) Proximal events in Wnt signal transduction. Nature Reviews 10, 468–477 - PubMed
-
- van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 - PubMed
-
- Yang J., Brown M. S., Liang G., Grishin N. V., Goldstein J. L. (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132, 387–396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

