A highly conserved cytoplasmic cysteine residue in the α4 nicotinic acetylcholine receptor is palmitoylated and regulates protein expression
- PMID: 22593584
- PMCID: PMC3391155
- DOI: 10.1074/jbc.M111.328294
A highly conserved cytoplasmic cysteine residue in the α4 nicotinic acetylcholine receptor is palmitoylated and regulates protein expression
Abstract
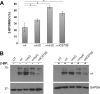
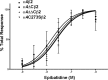
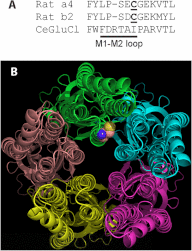
Nicotinic acetylcholine receptor (nAChR) cell surface expression levels are modulated during nicotine dependence and multiple disorders of the nervous system, but the mechanisms underlying nAChR trafficking remain unclear. To determine the role of cysteine residues, including their palmitoylation, on neuronal α4 nAChR subunit maturation and cell surface trafficking, the cysteines in the two intracellular regions of the receptor were replaced with serines using site-directed mutagenesis. Palmitoylation is a post-translational modification that regulates membrane receptor trafficking and function. Metabolic labeling with [(3)H]palmitate determined that the cysteine in the cytoplasmic loop between transmembrane domains 1 and 2 (M1-M2) is palmitoylated. When this cysteine is mutated to a serine, producing a depalmitoylated α4 nAChR, total protein expression decreases, but surface expression increases compared with wild-type α4 levels, as determined by Western blotting and enzyme-linked immunoassays, respectively. The cysteines in the M3-M4 cytoplasmic loop do not appear to be palmitoylated, but replacing all of the cysteines in the loop with serines increases total and cell surface expression. When all of the intracellular cysteines in both loops are mutated to serines, there is no change in total expression, but there is an increase in surface expression. Calcium accumulation assays and high affinity binding for [(3)H]epibatidine determined that all mutants retain functional activity. Thus, our results identify a novel palmitoylation site on cysteine 273 in the M1-M2 loop of the α4 nAChR and determine that cysteines in both intracellular loops are regulatory factors in total and cell surface protein expression of the α4β2 nAChR.
Figures



References
-
- Millar N. S. (2003) Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem. Soc. Trans. 31, 869–874 - PubMed
-
- Charollais J., Van Der Goot F. G. (2009) Palmitoylation of membrane proteins (Review). Mol. Membr. Biol. 26, 55–66 - PubMed
-
- el-Husseini Ael-D., Bredt D. S. (2002) Protein palmitoylation: A regulator of neuronal development and function. Nat. Rev. Neurosci. 3, 791–802 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

