Enhanced anti-serpin antibody activity inhibits autoimmune inflammation in type 1 diabetes
- PMID: 22593614
- PMCID: PMC3370061
- DOI: 10.4049/jimmunol.1200467
Enhanced anti-serpin antibody activity inhibits autoimmune inflammation in type 1 diabetes
Abstract
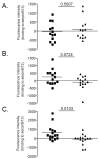
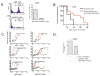
Intracellular (clade B) OVA-serpin protease inhibitors play an important role in tissue homeostasis by protecting cells from death in response to hypo-osmotic stress, heat shock, and other stimuli. It is not known whether these serpins influence immunological tolerance and the risk for autoimmune diseases. We found that a fraction of young autoimmune diabetes-prone NOD mice had elevated levels of autoantibodies against a member of clade B family known as serpinB13. High levels of anti-serpinB13 Abs were accompanied by low levels of anti-insulin autoantibodies, reduced numbers of islet-associated T cells, and delayed onset of diabetes. Exposure to anti-serpinB13 mAb alone also decreased islet inflammation, and coadministration of this reagent and a suboptimal dose of anti-CD3 mAb accelerated recovery from diabetes. In a fashion similar to that discovered in the NOD model, a deficiency in humoral activity against serpinB13 was associated with early onset of human type 1 diabetes. These findings suggest that, in addition to limiting exposure to proteases within the cell, clade B serpins help to maintain homeostasis by inducing protective humoral immunity.
Figures

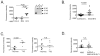




References
-
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Ann Rev Immunol. 2005;23:447–485. - PubMed
-
- Solomon M, Sarvetnick N. The pathogenesis of diabetes in the NOD mouse. Adv Immunol. 2004;84:239–264. - PubMed
-
- Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzji JG, Roep BO. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Eng J Med. 2001;345:1036–1040. - PubMed
-
- Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. - PubMed
-
- Patterson CC, Dahlquist GG, Gyurus A, Green A, Soltesz H EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-2020: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

