Optimization of acid protease production by Aspergillus niger I1 on shrimp peptone using statistical experimental design
- PMID: 22593695
- PMCID: PMC3349213
- DOI: 10.1100/2012/564932
Optimization of acid protease production by Aspergillus niger I1 on shrimp peptone using statistical experimental design
Abstract
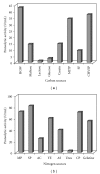
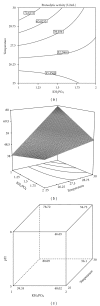
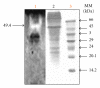
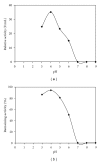
Medium composition and culture conditions for the acid protease production by Aspergillus niger I1 were optimized by response surface methodology (RSM). A significant influence of temperature, KH(2)PO(4), and initial pH on the protease production was evaluated by Plackett-Burman design (PBD). These factors were further optimized using Box-Behnken design and RSM. Under the proposed optimized conditions, the experimental protease production (183.13 U mL(-1)) closely matched the yield predicted by the statistical model (172.57 U mL(-1)) with R(2) = 0.914. Compared with the initial M1 medium on which protease production was 43.13 U mL(-1), a successful and significant improvement by 4.25 folds was achieved in the optimized medium containing (g/L): hulled grain of wheat (HGW) 5.0; KH(2)PO(4) 1.0; NaCl 0.3; MgSO(4)(7H(2)O) 0.5; CaCl(2) (7H(2)O) 0.4; ZnSO(4) 0.1; Na(2)HPO(4) 1.6; shrimp peptone (SP) 1.0. The pH was adjusted at 5 and the temperature at 30°C. More interestingly, the optimization was accomplished using two cheap and local fermentation substrates, HGW and SP, which may result in a significant reduction in the cost of medium constituents.
Figures
Similar articles
-
Low-cost fermentation medium for alkaline protease production by Bacillus mojavensis A21 using hulled grain of wheat and sardinella peptone.J Biosci Bioeng. 2010 Sep;110(3):288-94. doi: 10.1016/j.jbiosc.2010.03.015. Epub 2010 May 10. J Biosci Bioeng. 2010. PMID: 20547353
-
Optimization of alkaline protease production by Aspergillus clavatus ES1 in Mirabilis jalapa tuber powder using statistical experimental design.Appl Microbiol Biotechnol. 2008 Jul;79(6):915-23. doi: 10.1007/s00253-008-1508-0. Epub 2008 May 15. Appl Microbiol Biotechnol. 2008. PMID: 18481054
-
Improvement of xylanase production by Aspergillus niger XY-1 using response surface methodology for optimizing the medium composition.J Zhejiang Univ Sci B. 2008 Jul;9(7):558-66. doi: 10.1631/jzus.B0820038. J Zhejiang Univ Sci B. 2008. PMID: 18600786 Free PMC article.
-
Response surface analysis for the production of an enantioselective lipase from Aspergillus niger by solid-state fermentation.J Microbiol. 2009 Oct;47(5):563-71. doi: 10.1007/s12275-008-0279-8. Epub 2009 Oct 24. J Microbiol. 2009. PMID: 19851729
-
Medium optimization of antifungal activity production by Bacillus amyloliquefaciens using statistical experimental design.Prep Biochem Biotechnol. 2012;42(3):267-78. doi: 10.1080/10826068.2011.614989. Prep Biochem Biotechnol. 2012. PMID: 22509851
Cited by
-
Statistical optimization of fibrinolytic enzyme production using agroresidues by Bacillus cereus IND1 and its thrombolytic activity in vitro.Biomed Res Int. 2014;2014:725064. doi: 10.1155/2014/725064. Epub 2014 Jun 9. Biomed Res Int. 2014. PMID: 25003130 Free PMC article.
-
Statistical optimization of process parameters for lipase-catalyzed synthesis of triethanolamine-based esterquats using response surface methodology in 2-liter bioreactor.ScientificWorldJournal. 2013 Nov 10;2013:962083. doi: 10.1155/2013/962083. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24324389 Free PMC article.
-
Novel Sequential Screening and Enhanced Production of Fibrinolytic Enzyme by Bacillus sp. IND12 Using Response Surface Methodology in Solid-State Fermentation.Biomed Res Int. 2017;2017:3909657. doi: 10.1155/2017/3909657. Epub 2017 Jan 17. Biomed Res Int. 2017. PMID: 28321408 Free PMC article.
-
Desired soy sauce characteristics and autolysis of Aspergillus oryzae induced by low temperature conditions during initial moromi fermentation.J Food Sci Technol. 2019 Jun;56(6):2888-2898. doi: 10.1007/s13197-019-03742-5. Epub 2019 Apr 29. J Food Sci Technol. 2019. PMID: 31205344 Free PMC article.
-
Bio-prospecting of cuttle fish waste and cow dung for the production of fibrinolytic enzyme from Bacillus cereus IND5 in solid state fermentation.3 Biotech. 2016 Dec;6(2):231. doi: 10.1007/s13205-016-0553-0. Epub 2016 Oct 28. 3 Biotech. 2016. PMID: 28330303 Free PMC article.
References
-
- Banerjee UC, Sani RK, Azmi W, Soni R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochemistry. 1999;35(1-2):213–219.
-
- Gupta R, Beg QK, Khan S, Chauhan B. An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Applied Microbiology and Biotechnology. 2003;60(4):381–395. - PubMed
-
- Mitchell DA, Lonsane BK. Definition, characteristics and potential in solid state cultivation. In: Doelle HW, Mitchell SA, Rolz CE, editors. Applied Biotechnology Series. Amsterdam, The Netherlands: Elsevier; 1992. pp. 1–16.
-
- Laxman RS, Sonawane AP, More SV, et al. Optimization and scale up of production of alkaline protease from Conidiobolus coronatus . Process Biochemistry. 2005;40(9):3152–3158.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

