Lymphotoxin-beta receptor blockade induces inflammation and fibrosis in tolerized cardiac allografts
- PMID: 22594431
- PMCID: PMC3424360
- DOI: 10.1111/j.1600-6143.2012.04090.x
Lymphotoxin-beta receptor blockade induces inflammation and fibrosis in tolerized cardiac allografts
Abstract
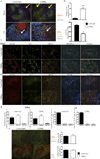
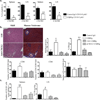
The lymphotoxin system (LT) regulates interactions between lymphocytes and stromal cells to maintain lymphoid microenvironmental homeostasis. Soluble LT beta-receptor-Ig (LTβRIg) blocks lymphocyte LTα1β2-stromal cell LTβR signaling. In a murine cardiac allograft model, LTbRIg treatment reversed the tolerance induced by anti-CD40L antibody leading to graft inflammation and fibrosis. LTβRIg treatment decreased PD-L1 expression by blood endothelial cells, and decreased VCAM-1 while increasing CXCL1, CXCL2, CXCL12, CCL5, CCL21 and IL-6 expression in fibroblastic reticular cells. In secondary lymphoid organs these effects caused T- and B cell zone disruption, loss of CD35(+) follicular dendritic cells and abnormal recruitment of CD11b(+) Ly6G(+) neutrophils. These disruptions correlated with increased numbers of CD8(+) T cells and CD11b(+) Ly6G(+) neutrophils, and decreased numbers of CD4(+) T cells and Foxp3(+) regulatory T cells in the grafts. Depleting neutrophils or blocking neutrophil-attracting chemokines restored normal histology in lymph node, spleen and grafts. Taken together, LTβRIg treatment altered stromal subset, particularly fibroblastic reticular cell, production of cytokines and chemokines, resulting in changes in neutrophil recruitment in spleen, lymph node and grafts, and inflammation and fibrosis associated with decreased Foxp3(+) regulatory T cells and increased CD8(+) T cell infiltration of grafts.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures




References
-
- Bashuda H, Shimizu A, Uchiyama M, Okumura K. Prolongation of renal allograft survival by anergic cells: advantages and limitations. Clin Transplant. 2010;24(Suppl 22):6–10. - PubMed
-
- Watson D, Hu M, Zhang GY, Wang YM, Alexander SI. Tolerance induction by removal of alloreactive T cells: in-vivo and pruning strategies. Curr Opin Organ Transplant. 2009;14(4):357–363. - PubMed
-
- Gudmundsdottir H, Turka LA. T cell costimulatory blockade: new therapies for transplant rejection. J Am Soc Nephrol. 1999;10(6):1356–1365. - PubMed
-
- Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14(5):592–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

