Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy
- PMID: 22594993
- PMCID: PMC3510308
- DOI: 10.1111/j.1600-6143.2012.04087.x
Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy
Abstract
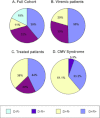
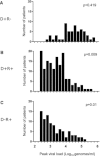
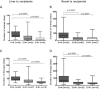
After allotransplantation, cytomegalovirus (CMV) may be transmitted from the donor organ, giving rise to primary infection in a CMV negative recipient or reinfection in one who is CMV positive. In addition, latent CMV may reactivate in a CMV positive recipient. In this study, serial blood samples from 689 kidney or liver transplant recipients were tested for CMV DNA by quantitative PCR. CMV was managed using preemptive antiviral therapy and no patient received antiviral prophylaxis. Dynamic and quantitative measures of viremia and treatment were assessed. Median peak viral load, duration of viremia and duration of treatment were highest during primary infection, followed by reinfection then reactivation. In patients who experienced a second episode of viremia, the viral replication rate was significantly slower than in the first episode. Our data provide a clear demonstration of the immune control of CMV in immunosuppressed patients and emphasize the effectiveness of the preemptive approach for prevention of CMV syndrome and end organ disease. Overall, our findings provide quantitative biomarkers which can be used in pharmacodynamic assessments of the ability of novel CMV vaccines or antiviral drugs to reduce or even interrupt such transmission.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures


References
-
- Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. - PubMed
-
- Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. - PubMed
-
- Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228–1237. - PubMed
-
- Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000;356:645–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

