MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice
- PMID: 22595192
- PMCID: PMC7106219
- DOI: 10.1016/j.intimp.2012.05.003
MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice
Abstract
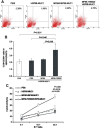
MF59 is an oil-in-water emulsion adjuvant approved for influenza vaccines for human use in Europe. Due to its Th2 inducing properties, MF59 is seldom tested for cancer vaccines. In this study, MF59 formulated with a C-type CpG oligodeoxynucleotide (YW002) was tested for its Th1 adjuvant activity to induce immune responses to HSP65-MUC1, a recombinant fusion protein incorporating a mycobacterial heat shock protein (HSP65) and mucin 1, cell surface associated (MUC1) derived peptide. Combination of YW002 with MF59 (MF59-YW002) could confer a potent Th1 biasing property to the adjuvant, which enhanced the immunogenicity of HSP65-MUC1 to induce significantly higher levels of specific IgG2c, increased IFN-γ mRNA expression in splenocytes and the generation of antigen-specific cytotoxic T lymphocytes in mice. When prophylactically applied, MF59-YW002 adjuvant containing HSP65-MUC1 inhibited the growth of MUC1+ B16 melanoma and prolonged the survival of tumor-bearing mice. In contrast, adjuvant containing MF59 with HSP65-MUC1 in the absence of YW002, promoted the growth of MUC1+ B16 melanoma in mice. These results suggest that MF59 plus CpG oligodeoxynucleotide might be developed as an efficient adjuvant for tumor vaccines against melanoma, and possibly other tumors.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures






References
-
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. - PubMed
-
- Dubensky T.W., Jr., Reed S.G. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155–161. - PubMed
-
- Clark T.W., Pareek M., Hoschler K., Dillon H., Nicholson K.G., Groth N. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361(25):2424–2435. - PubMed
-
- Esposito S., D'Angelo E., Daleno C., Peia F., Scala A., Serra D. Immunogenicity, safety and tolerability of monovalent 2009 pandemic influenza A/H1N1 MF59-adjuvanted vaccine in patients with β-thalassemia major. Vaccine. 2010;28(50):7825–7828. - PubMed
-
- Esposito S., Selicorni A., Daleno C., Valzano A., Cerutti M., Galeone C. Immunogenicity, safety and tolerability of monovalent 2009 pandemic influenza A/H1N1 MF59-adjuvanted vaccine in children and adolescents with Williams or Cornelia De Lange syndrome. Hum Vaccin. 2011;7(6):613–617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

