Rescue of misrouted GnRHR mutants reveals its constitutive activity
- PMID: 22595961
- PMCID: PMC3385788
- DOI: 10.1210/me.2012-1089
Rescue of misrouted GnRHR mutants reveals its constitutive activity
Abstract
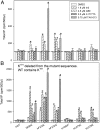
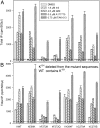
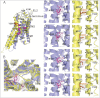
G protein-coupled receptors (GPCR) play central roles in almost all physiological functions, and mutations in GPCR are responsible for over 30 hereditary diseases associated with loss or gain of receptor function. Gain of function mutants are frequently described as having constitutive activity (CA), that is, they activate effectors in the absence of agonist occupancy. Although many GPCR have mutants with CA, the GnRH receptor (GnRHR) was not, until 2010, associated with any CA mutants. The explanation for the failure to observe CA appears to be that the quality control system of the cell recognizes CA mutants of GnRHR as misfolded and retains them in the endoplasmic reticulum. In the present study, we identified several human (h)GnRHR mutants with substitutions in transmembrane helix 6 (F(272)K, F(272)Q, Y(284)F, C(279)A, and C(279)S) that demonstrate varying levels of CA after being rescued by pharmacoperones from different chemical classes and/or deletion of residue K(191), a modification that increases trafficking to the plasma membrane. The movement of the mutants from the endoplasmic reticulum (unrescued) to the plasma membrane (after rescue) is supported by confocal microscopy. Judging from the receptor-stimulated inositol phosphate production, mutants F(272)K and F(272)Q, after rescue, display the largest level of CA, an amount that is comparable with agonist-stimulated activation. Because mutations in other GPCR are, like the hGnRHR, scrutinized by the quality control system, this general approach may reveal CA in receptor mutants from other systems. A computer model of the hGnRHR and these mutants was used to evaluate the conformation associated with CA.
Figures


Similar articles
-
Use of pharmacoperones to reveal GPCR structural changes associated with constitutive activation and trafficking.Methods Enzymol. 2010;485:277-92. doi: 10.1016/B978-0-12-381296-4.00016-6. Methods Enzymol. 2010. PMID: 21050923
-
Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect.Mol Endocrinol. 2004 Jul;18(7):1787-97. doi: 10.1210/me.2004-0091. Epub 2004 Apr 22. Mol Endocrinol. 2004. PMID: 15105440
-
Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor.Mol Endocrinol. 2009 Feb;23(2):157-68. doi: 10.1210/me.2008-0384. Epub 2008 Dec 18. Mol Endocrinol. 2009. PMID: 19095769 Free PMC article.
-
Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs.Trends Endocrinol Metab. 2010 Mar;21(3):190-7. doi: 10.1016/j.tem.2009.11.003. Epub 2009 Dec 11. Trends Endocrinol Metab. 2010. PMID: 20005736 Free PMC article. Review.
-
Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease.Mol Cell Endocrinol. 2009 Feb 27;299(2):137-45. doi: 10.1016/j.mce.2008.10.051. Epub 2008 Nov 18. Mol Cell Endocrinol. 2009. PMID: 19059461 Free PMC article. Review.
Cited by
-
Transitioning pharmacoperones to therapeutic use: in vivo proof-of-principle and design of high throughput screens.Pharmacol Res. 2014 May;83:38-51. doi: 10.1016/j.phrs.2013.12.004. Epub 2013 Dec 25. Pharmacol Res. 2014. PMID: 24373832 Free PMC article. Review.
-
Gonadotropin-Releasing Hormone Receptor (GnRHR) and Hypogonadotropic Hypogonadism.Int J Mol Sci. 2023 Nov 4;24(21):15965. doi: 10.3390/ijms242115965. Int J Mol Sci. 2023. PMID: 37958948 Free PMC article. Review.
-
Functional relevance of naturally occurring mutations in adhesion G protein-coupled receptor ADGRD1 (GPR133).BMC Genomics. 2016 Aug 11;17(1):609. doi: 10.1186/s12864-016-2937-2. BMC Genomics. 2016. PMID: 27516204 Free PMC article.
-
Rescue of mutant gonadotropin-releasing hormone receptor function independent of cognate receptor activity.Sci Rep. 2020 Jun 29;10(1):10579. doi: 10.1038/s41598-020-67473-w. Sci Rep. 2020. PMID: 32601341 Free PMC article.
-
Pharmacoperone rescue of vasopressin 2 receptor mutants reveals unexpected constitutive activity and coupling bias.PLoS One. 2017 Aug 2;12(8):e0181830. doi: 10.1371/journal.pone.0181830. eCollection 2017. PLoS One. 2017. PMID: 28767678 Free PMC article.
References
-
- Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. 2003. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305:608–614 - PubMed
-
- Brothers SP, Cornea A, Janovick JA, Conn PM. 2004. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol 18:1787–1797 - PubMed
-
- Janovick JA, Maya-Nunez G, Conn PM. 2002. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87:3255–3262 - PubMed

