WHIM syndrome caused by a single amino acid substitution in the carboxy-tail of chemokine receptor CXCR4
- PMID: 22596258
- PMCID: PMC3390956
- DOI: 10.1182/blood-2011-12-395608
WHIM syndrome caused by a single amino acid substitution in the carboxy-tail of chemokine receptor CXCR4
Abstract
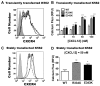
WHIM syndrome is a rare, autosomal dominant, immunodeficiency disorder so-named because it is characterized by warts, hypogammaglobulinemia, infections, and myelokathexis (defective neutrophil egress from the BM). Gain-of-function mutations that truncate the C-terminus of the chemokine receptor CXCR4 by 10-19 amino acids cause WHIM syndrome. We have identified a family with autosomal dominant inheritance of WHIM syndrome that is caused by a missense mutation in CXCR4, E343K (1027G → A). This mutation is also located in the C-terminal domain, a region responsible for negative regulation of the receptor. Accordingly, like CXCR4(R334X), the most common truncation mutation in WHIM syndrome, CXCR4(E343K) mediated approximately 2-fold increased signaling in calcium flux and chemotaxis assays relative to wild-type CXCR4; however, CXCR4(E343K) had a reduced effect on blocking normal receptor down-regulation from the cell surface. Therefore, in addition to truncating mutations in the C-terminal domain of CXCR4, WHIM syndrome may be caused by a single charge-changing amino acid substitution in this domain, E343K, that results in increased receptor signaling.
Figures




References
-
- Gorlin RJ, Gelb B, Diaz GA, et al. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91(5):368–376. - PubMed
-
- Wetzler M, Talpaz M, Kleinerman ES, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89(5):663–672. - PubMed
-
- Dotta L, Tassone L, Badolato R. Clinical and genetic features of warts, hypogammaglobulinemia, infections and myelokathexis (WHIM) syndrome. Curr Mol Med. 2011;11(4):317–325. - PubMed
-
- Tassone L, Moratto D, Vermi W, et al. Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood. 2010;116(23):4870–4873. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

