Generation and external validation of a tumor-derived 5-gene prognostic signature for recurrence of lymph node-negative, invasive colorectal carcinoma
- PMID: 22605513
- PMCID: PMC3532613
- DOI: 10.1002/cncr.27628
Generation and external validation of a tumor-derived 5-gene prognostic signature for recurrence of lymph node-negative, invasive colorectal carcinoma
Abstract
Background: One in 4 patients with lymph node-negative, invasive colorectal carcinoma (CRC) develops recurrent disease after undergoing curative surgery, and most die of advanced disease. Predicting which patients will develop a recurrence is a significantly growing, unmet medical need.
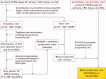
Methods: Archival formalin-fixed, paraffin-embedded (FFPE) primary adenocarcinoma tissues obtained at surgery were retrieved from 74 patients with CRC (15 with stage I disease and 59 with stage II disease) for Training/Test Sets. In addition, FFPE tissues were retrieved from 49 patients with stage I CRC and 215 patients with stage II colon cancer for an External Validation (EV) Set (n = 264) from 18 hospitals in 4 countries. No patients had received neoadjuvant/adjuvant therapy. Proprietary genetic programming analysis of expression profiles for 225 prespecified tumor genes was used to create a 36-month recurrence risk signature.
Results: Using reverse transcriptase-polymerase chain reaction, a 5-gene rule correctly classified 62 of 92 recurrent patients and 87 of 172 nonrecurrent patients in the EV Set (sensitivity, 0.67; specificity, 0.51). "High-risk" patients had a greater probability of 36-month recurrence (42%) than "low-risk" patients (26%; hazard ratio, 1.80; 95% confidence interval, 1.19-2.71; P = .007; Cox regression) independent of T-classification, the number of lymph nodes examined, histologic grade/subtype, anatomic location, age, sex, or race. The rule outperformed (P = .021) current National Comprehensive Cancer Network Guidelines (hazard ratio, 0.897). The same rule also differentiated the risk of recurrence (hazard ratio, 1.63; P = .031) in a subset of patients from the EV Set who had stage I/II colon cancer only (n = 251).
Conclusions: To the authors' knowledge, the 5-gene rule (OncoDefender-CRC) is the first molecular prognostic that has been validated in both stage I CRC and stage II colon cancer. It outperforms standard clinicopathologic prognostic criteria and obviates the need to retrieve ≥12 lymph nodes for accurate prognostication. It identifies those patients most likely to develop recurrent disease within 3 years after curative surgery and, thus, those most likely to benefit from adjuvant treatment.
Copyright © 2012 American Cancer Society.
Figures


References
-
- Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–385. - PubMed
-
- American Joint Committee on Cancer (AJCC) Colon and rectum. In: Edge SB, Byrd DR, Compton CC, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. pp. 143–164.
-
- Lines LM, Lang K, Lee DW, et al. Trends in stage distribution and survival among elderly colorectal cancer patients in the US [abstract] J Clin Oncol. 2008;26(May 20 suppl) Abstract 4043.
-
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines)—Colon Cancer (V1.2012) and Rectal Cancer (V1.2012). Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed October 10, 2011. - PubMed
-
- Obrand DI, Gordan PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

