Structural and functional characterization of two alternative splicing variants of mouse Endothelial Cell-Specific Chemotaxis Regulator (ECSCR)
- PMID: 22606020
- PMCID: PMC3344256
- DOI: 10.3390/ijms13044920
Structural and functional characterization of two alternative splicing variants of mouse Endothelial Cell-Specific Chemotaxis Regulator (ECSCR)
Abstract
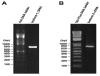
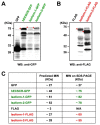
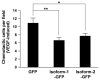
Endothelial cells (ECs) that line the lumen of blood vessels are important players in blood vessel formation, and EC migration is a key component of the angiogenic process. Thus, identification of genes that are specifically or preferentially expressed in vascular ECs and in-depth understanding of their biological functions may lead to discovery of new therapeutic targets. We have previously reported molecular characterization of human endothelial cell-specific molecule 2 (ECSM2)/endothelial cell-specific chemotaxis regulator (ECSCR). In the present study, we cloned two mouse full-length cDNAs by RT-PCR, which encode two putative ECSCR isoform precursors with considerable homology to the human ECSCR. Nucleotide sequence and exon-intron junction analyses suggested that they are alternative splicing variants (ECSCR isoform-1 and -2), differing from each other in the first and second exons. Quantitative RT-PCR results revealed that isoform-2 is the predominant form, which was most abundant in heart, lung, and muscles, and moderately abundant in uterus and testis. In contrast, the expression of isoform-1 seemed to be more enriched in testis. To further explore their potential cellular functions, we expressed GFP- and FLAG-tagged ECSCR isoforms, respectively, in an ECSCR deficient cell line (HEK293). Interestingly, the actual sizes of either ECSCR-GFP or -FLAG fusion proteins detected by immunoblotting are much larger than their predicted sizes, suggesting that both isoforms are glycoproteins. Fluorescence microscopy revealed that both ECSCR isoforms are localized at the cell surface, which is consistent with the structural prediction. Finally, we performed cell migration assays using mouse endothelial MS1 cells overexpressing GFP alone, isoform-1-GFP, and isoform-2-GFP, respectively. Our results showed that both isoforms significantly inhibited vascular epidermal growth factor (VEGF)-induced cell migration. Taken together, we have provided several lines of experimental evidence that two mouse ECSCR splicing variants/isoform precursors exist. They are differentially expressed in a variety of tissue types and likely involved in modulation of vascular EC migration. We have also defined the gene structure of mouse ECSCR using bioinformatics tools, which provides new information towards a better understanding of alternative splicing of ECSCR.
Keywords: ECSCR/ECSM2; alternative splicing; cDNA cloning and expression; endothelial cell migration; exon-intron boundary; gene structure; isoform.
Figures





References
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Folkman J., D’Amore P.A. Blood vessel formation: What is its molecular basis? Cell. 1996;87:1153–1155. - PubMed
-
- Jacobs J. Combating cardiovascular disease with angiogenic therapy. Drug Discov. Today. 2007;12:1040–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

