Human Muscle Progenitor Cells Displayed Immunosuppressive Effect through Galectin-1 and Semaphorin-3A
- PMID: 22606205
- PMCID: PMC3347758
- DOI: 10.1155/2012/412610
Human Muscle Progenitor Cells Displayed Immunosuppressive Effect through Galectin-1 and Semaphorin-3A
Abstract
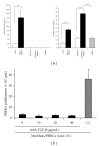
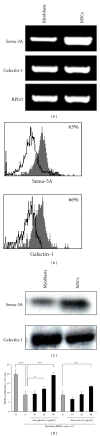
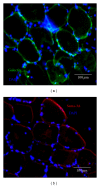
In human skeletal muscle, myoblasts represent the main population of myogenic progenitors. We previously showed that, beside their myogenic differentiation capacities, myoblasts also differentiate towards osteogenic and chondrogenic lineages, some properties generally considered being hallmarks of mesenchymal stem cells (MSCs). MSCs are also characterized by their immunosuppressive potential, through cell-cell contacts and soluble factors, including prostaglandin E-2 (PGE-2), transforming growth factor-β1 (TGF-β1), interleukine-10, or indoleamine 2,3-dioxygenase. We and others also reported that Galectin-1 (Gal-1) and Semaphorin-3A (Sema-3A) were involved in MSCs-mediated immunosuppression. Here, we show that human myoblasts induce a significant and dose-dependant proliferation inhibition, independently of PGE-2 and TGF-β1. Our experiments revealed that myoblasts, in culture or in situ in human muscles, expressed and secreted Gal-1 and Sema-3A. Furthermore, myoblasts immunosuppressive functions were reverted by using blocking antibodies against Gal-1 or Sema-3A. Together, these results demonstrate an unsuspected immunosuppressive effect of myoblasts that may open new therapeutic perspectives.
Figures

References
-
- Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat ML, Gabbiani G, Bader CR. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60(1):47–57. - PubMed
-
- Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. - PubMed
-
- Sinanan ACM, Hunt NP, Lewis MP. Human adult craniofacial muscle-derived cells: neural-cell-adhesion- molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnology and Applied Biochemistry. 2004;40(1):25–34. - PubMed
-
- Lecourt S, Marolleau JP, Fromigué O, et al. Characterization of distinct mesenchymal-like cell populations from human skeletal muscle in situ and in vitro. Experimental Cell Research. 2010;316(15):2513–2526. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

