Glucose-dependent regulation of NR2F2 promoter and influence of SNP-rs3743462 on whole body insulin sensitivity
- PMID: 22606236
- PMCID: PMC3351448
- DOI: 10.1371/journal.pone.0035810
Glucose-dependent regulation of NR2F2 promoter and influence of SNP-rs3743462 on whole body insulin sensitivity
Abstract
Background: The Nuclear Receptor 2F2 (NR2F2/COUP-TFII) heterozygous knockout mice display low basal insulinemia and enhanced insulin sensitivity. We previously established that insulin represses NR2F2 gene expression in pancreatic β-cells. The cis-regulatory region of the NR2F2 promoter is unknown and its influence on metabolism in humans is poorly understood. The present study aimed to identify the regulatory regions that control NR2F2 gene transcription and to evaluate the effect of NR2F2 promoter variation on glucose homeostasis in humans.
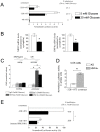
Methodology/principal findings: Regulation of the NR2F2 promoter was assessed using gene reporter assays, ChIP and gel shift experiments. The effects of variation at SNP rs3743462 in NR2F2 on quantitative metabolic traits were studied in two European prospective cohorts. We identified a minimal promoter region that down-regulates NR2F2 expression by attenuating HNF4α activation in response to high glucose concentrations. Subjects of the French DESIR population, who carried the rs3743462 T-to-C polymorphism, located in the distal glucose-responsive promoter, displayed lower basal insulin levels and lower HOMA-IR index. The C-allele at rs3743462 was associated with increased NR2F2 binding and decreased NR2F2 gene expression.
Conclusions/significance: The rs3743462 polymorphism affects glucose-responsive NR2F2 promoter regulation and thereby may influence whole-body insulin sensitivity, suggesting a role of NR2F2 in the control of glucose homeostasis in humans.
Conflict of interest statement
Figures

References
-
- Bardoux P, Zhang P, Flamez D, Perilhou A, Lavin T, et al. Diabetes; 2005. Essential role of chicken ovalbumin upstream promoter-transcription factor II in insulin secretion and insulin sensitivity revealed by conditional gene knockout. pp. 1357–1363. - PubMed
-
- Myers S, Wang S-C, Muscat G. The chicken ovalbumin upstream promoter-transcription factors modulate genes and pathways involved in skeletal muscle cell metabolism. J Biol Chem. 2006;281:24149–24160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

