A multi-platform flow device for microbial (co-) cultivation and microscopic analysis
- PMID: 22606321
- PMCID: PMC3351485
- DOI: 10.1371/journal.pone.0036982
A multi-platform flow device for microbial (co-) cultivation and microscopic analysis
Abstract
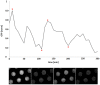
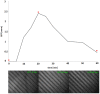
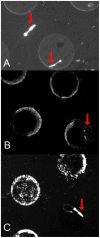
Novel microbial cultivation platforms are of increasing interest to researchers in academia and industry. The development of materials with specialized chemical and geometric properties has opened up new possibilities in the study of previously unculturable microorganisms and has facilitated the design of elegant, high-throughput experimental set-ups. Within the context of the international Genetically Engineered Machine (iGEM) competition, we set out to design, manufacture, and implement a flow device that can accommodate multiple growth platforms, that is, a silicon nitride based microsieve and a porous aluminium oxide based microdish. It provides control over (co-)culturing conditions similar to a chemostat, while allowing organisms to be observed microscopically. The device was designed to be affordable, reusable, and above all, versatile. To test its functionality and general utility, we performed multiple experiments with Escherichia coli cells harboring synthetic gene circuits and were able to quantitatively study emerging expression dynamics in real-time via fluorescence microscopy. Furthermore, we demonstrated that the device provides a unique environment for the cultivation of nematodes, suggesting that the device could also prove useful in microscopy studies of multicellular microorganisms.
Conflict of interest statement
Figures



Similar articles
-
Pumpless platform for high-throughput dynamic multicellular culture and chemosensitivity evaluation.Lab Chip. 2019 Jan 15;19(2):254-261. doi: 10.1039/c8lc00872h. Lab Chip. 2019. PMID: 30547180 Free PMC article.
-
A microfluidic co-cultivation platform to investigate microbial interactions at defined microenvironments.Lab Chip. 2018 Dec 18;19(1):98-110. doi: 10.1039/c8lc00977e. Lab Chip. 2018. PMID: 30488920
-
A PMMA microfluidic droplet platform for in vitro protein expression using crude E. coli S30 extract.Lab Chip. 2009 Dec 7;9(23):3391-8. doi: 10.1039/b911581a. Epub 2009 Oct 1. Lab Chip. 2009. PMID: 19904406
-
Pumps for microfluidic cell culture.Electrophoresis. 2014 Feb;35(2-3):245-57. doi: 10.1002/elps.201300205. Epub 2013 Oct 1. Electrophoresis. 2014. PMID: 23893649 Review.
-
Microfluidic systems for high-throughput and high-content screening using the nematode Caenorhabditis elegans.Lab Chip. 2017 Nov 7;17(22):3736-3759. doi: 10.1039/c7lc00509a. Lab Chip. 2017. PMID: 28840220 Review.
Cited by
-
Decolorization of Selected Synthetic Textile Dyes by Yeasts from Leaves and Fruit Peels.J Health Pollut. 2016 Jun 16;6(10):42-55. doi: 10.5696/2156-9614-6-10.42. eCollection 2016 Jun. J Health Pollut. 2016. PMID: 30524784 Free PMC article.
-
Physically Triggered Morphology Changes in a Novel Acremonium Isolate Cultivated in Precisely Engineered Microfabricated Environments.Front Microbiol. 2017 Jul 14;8:1269. doi: 10.3389/fmicb.2017.01269. eCollection 2017. Front Microbiol. 2017. PMID: 28769882 Free PMC article.
-
A Two-Compartment Fermentation System to Quantify Strain-Specific Interactions in Microbial Co-Cultures.Bioengineering (Basel). 2023 Jan 11;10(1):103. doi: 10.3390/bioengineering10010103. Bioengineering (Basel). 2023. PMID: 36671675 Free PMC article.
-
Co-culture systems and technologies: taking synthetic biology to the next level.J R Soc Interface. 2014 Jul 6;11(96):20140065. doi: 10.1098/rsif.2014.0065. J R Soc Interface. 2014. PMID: 24829281 Free PMC article. Review.
-
No man's land: Species-specific formation of exclusion zones bordering Actinomyces graevenitzii microcolonies in nanoliter cultures.Microbiologyopen. 2021 Jan;10(1):e1137. doi: 10.1002/mbo3.1137. Epub 2021 Feb 5. Microbiologyopen. 2021. PMID: 33544453 Free PMC article.
References
-
- Kaeberlein T, Lewis K, Epstein SS. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science. 2002;296:1127–1129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

